Ultrasound of the Month: Clot in Transit
/Case
A middle aged with a history of gunshot wounds (GSWs) to the abdomen, extremities, and head presents to the emergency department (ED) with syncope. He has been at inpatient rehabilitation (IPR) and then home for almost three weeks since being discharged. He states that he was ambulating out of the bathroom on the day of presentation and suddenly lost consciousness and awoke on the floor after an unknown period of time. He denies chest pain but admits to palpitations and shortness of breath at time of evaluation.
At the time of ED presentation, he was noted to be markedly tachycardic to the 160s and thus was evaluated in the resuscitation bay. He did not require any oxygen at the time of initial evaluation. His exam was significant for a generally chronically ill appearing male in mild distress with clear lungs and a soft abdomen. His G tube, J tube, and pancreatic drain sites appeared clean and dry. He was answering questions appropriately. His medication list did not reflect any anticoagulation and thus the providers had a strong suspicion for pulmonary embolism (PE) as a cause of his syncope, shortness of breath, and tachycardia.
Before the patient was able to be brought to the computed tomography (CT) scanner, he developed significant hypoxia requiring 15 liters/minute via a non-rebreather mask. His blood pressure remained within normal limits. Trauma and neurosurgery were preemptively contacted in anticipation of the patient requiring systemic anticoagulation and both services gave permission for this despite the multiple recent surgeries.
At this point, the patient was too unstable for the CT scanner. A cardiac echo was performed in the resuscitation bay.
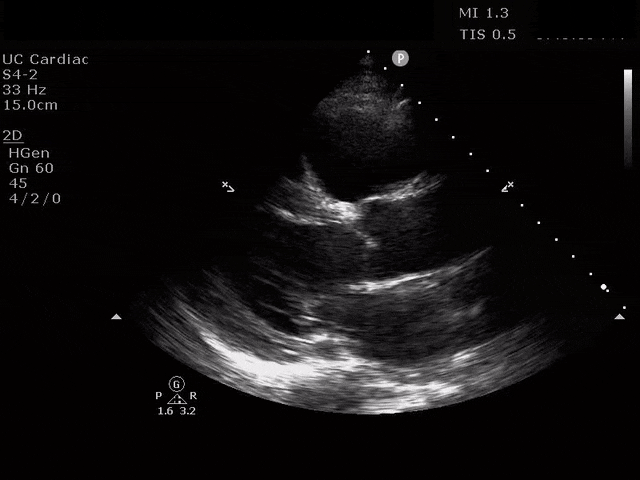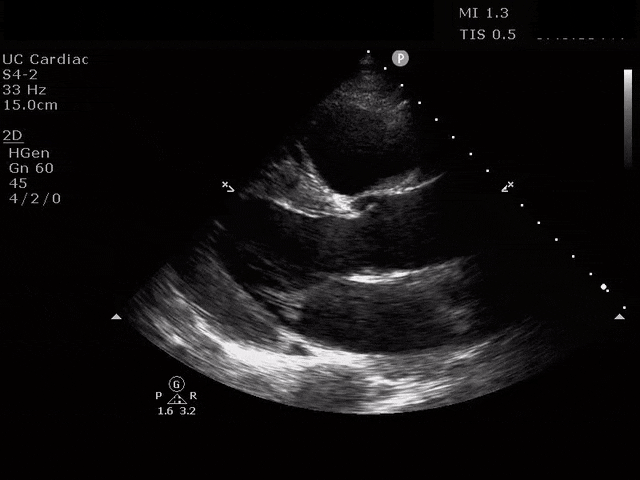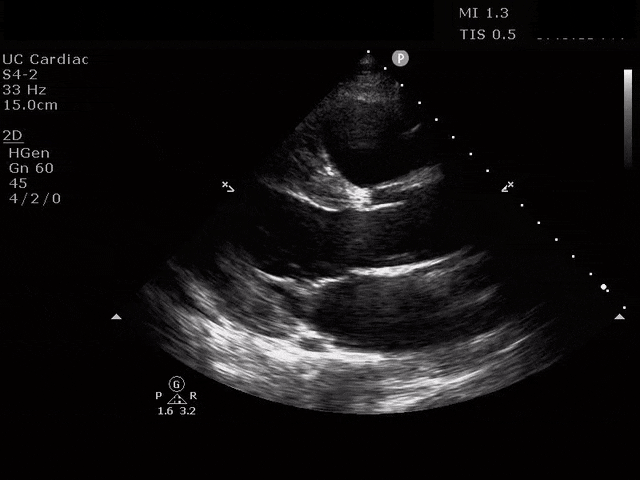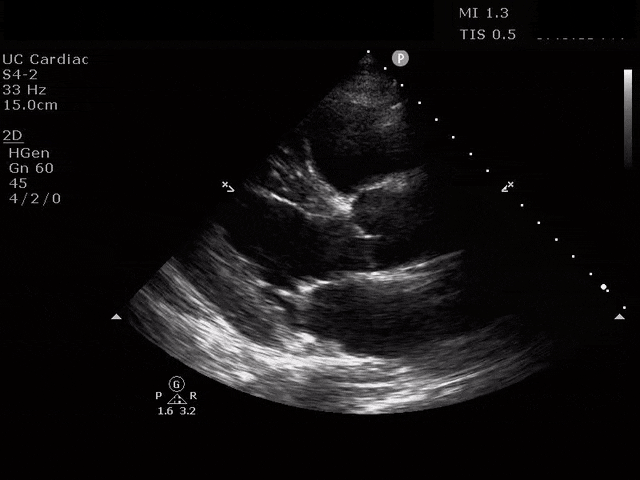
Parasternal Long Axis
Parasternal Short Axis
Apical 4 Chamber
The ultrasound clips above reveal signs of right heart strain, septal bowing, with a clearly visualized clot in the right atrium. What would your next step be? What kind of signs can we expect on a bedside echo that suggest PE and right heart strain?
We will delve into the remainder of the patient case after we discuss these echocardiographic findings.
Overall the sensitivity and specificity of point of care ultrasound (POCUS) to evaluate for PE and acute right heart strain are driven by pre-test probabilities. For example, ultrasound is highly sensitive for large, central PE’s in a patient with abnormal vital signs [1,2]. Here, we will outline some of the signs that emergency medicine physicians can look for quickly and easily at the bedside in a patient being evaluated for PE.
1. Increased right ventricle (RV) to left ventricle (LV) size ratio
As we know, the overall pressures of the right side of the heart are much lower than those of the left side. Normally, the visual ratio of the RV:LV should be 0.67:1 on the apical four chamber view [3]. A PE causes acute increase in the pressures of the right heart. Given the relatively thin, compliant wall of the RV, this increased RV afterload causes a rapid enlargement in size [4]. This acutely protends a worse outcome for patients, with higher overall mortality [5]. A good rule of thumb is that when the ratio of the RV:LV is greater than or equal to 1:1, the RV is dilated. If one were to measure these with calipers on the US machine, measure at end-diastole from the mid wall to the septum, when the chambers are at their largest [4]. Additionally, evaluation of the RV free wall thickness in the subxiphoid view may help you determine whether the RV dilation is acute or chronic. If the RV free wall thickness is >5mm at end-diastole, this suggests chronic RV hypertrophy [4, 6].
2. Abnormal septal wall motion
In the normal physiologic state, pressures within the left side of the heart, particularly the left ventricle, should always exceed those on the right side of the heart at any given moment. Because of this, the central area of the interventricular septum in a heart with normal physiology should always ‘bow’ into the right ventricle [4].
Septal dyskinesia refers to abnormal septal motion, like flattening or bowing of the septum into the LV. You can see this at end-diastole and end-systole. This appears most often with a large clot burden or shock [7]. The best way to assess this is in the parasternal short axis at the level of the papillary muscles (traditional parasternal short). One may see a “D-shaped” septum, most notable at end-systole when the delta pressure gradient is the largest between the RV and the LV [4,6]. Patients with chronic pulmonary hypertension may have paradoxical septal motion at baseline [8]. Additionally, in patients who have chronic dilation of the LV prior to any new insult caused by a PE, the septal motion may be normal given the balanced dilation of both ventricles [4].
3. McConnell’s sign
McConnell’s sign refers to diffuse hypokinesis of the RV with apical sparing. This finding is manifested as an apex that contracts well during systole. In terms of why this happens, some suggest that since the RV apex is tethered to the LV, it pulls inward when the LV is hyperdynamic [9]. Also recall that the apex is perfused by the right coronary artery and the left anterior descending artery, as opposed to the free wall which is perfused by the RCA alone, and may experience local ischemia due to increased wall stress. This sign is more specific for PE than it is sensitive, and this specificity seems to increase with higher heart rates [2, 9, 10]. A 2017 meta-analysis calculated the sensitivity of this finding to be 22% with specificity of 97% [11]. This sign is best assessed subjectively in the apical four chamber view though you can assess it objectively by measuring the free wall and apical movement in mm.
4. Tricuspid annular plane systolic excursion (TAPSE)
The emergency physician can and should perform a tricuspid annular plane systolic excursion to quantitatively assess the RV contraction. In fact, the longitudinal contraction seen here generates about 80% of the RV’s cardiac output [12]. A decreased TAPSE in PE is independently predictive of 30-day mortality in PE [13]. We obtain this view in the apical four chamber view at the true apex, visualizing the chambers in their longest cross section. The M-mode cursor is placed over the lateral tricuspid valve annulus, and the resultant “wave” shows the displacement of this annulus through systole and diastole [4]. A normal mean TAPSE is 24mm and the cutoff for abnormal TAPSE is <17mm [3]. One strength of the TAPSE is that among emergency physicians tasked with determining simply abnormal vs normal TAPSE, the interrater reliability agreement was quite high [14].
5. Tricuspid regurgitation
As the RV free wall dilates in the setting of increased pulmonary vascular resistance, the tricuspid valve (TV) annulus also dilates and can be used as a marker of RV strain [4]. The emergency physician may best be able to evaluate this in the apical four chamber view using color doppler over the TV and right atrium (RA). If a jet is present, the continuous wave doppler can be used to obtain the max velocity of the tricuspid regurgitation. An experienced user can then use this measurement to estimate the pulmonary artery systolic pressure. This can be difficult to obtain in the emergency department using POCUS. Additionally, it can be elevated in chronic RV strain as well, thus rendering it nondiagnostic for acute strain in this population subset [6].
There are additional, more complex methods for evaluating right heart strain at the bedside acutely such as evaluating for PA mid-systolic notching and the 60/60 sign [4]. More information can be found regarding advanced imaging via the resources below.
Back to the Case…
After the providers visualized the clot in transit in the RA, the patient suffered a cardiac arrest and eventually ROSC was obtained, inhaled nitric oxide was started and the providers discussed the possibility of thrombolysis. After pulses were lost again, tissue plasminogen activator (tPA) 50mg was administered, compressions and ACLS were continued. After several rounds of ACLS, ROSC was obtained and that patient was admitted to the ICU for ongoing care
Ultimately the patient’s CT pulmonary angiogram revealed a saddle PE and bilateral lower extremity duplexes revealed multiple DVTs. He underwent targeted temperature management in the MICU, and had meaningful neurologic recovery
AUTHORED BY SARAH WOLOCHATIUK, MD
Dr. Wolochatiuk (@sarah_wolo) is a PGY-4 in Emergency Medicine at the University of Cincinnati
PEER REVIEW BY PATRICK MINGES, MD AND JESSICA BAEZ, MD
Dr. Minges (@mingespg) is an Assistant Professor of Emergency Medicine and Ultrasound Faculty at the University of Cincinnati
Dr. Baez (@blonde_doctr) is an Assistant Professor of Emergency Medicine at the University of Cincinnati and Assistant Residency Director.
EDITING AND LAYOUT BY ARTHUR BROADSTOCK, MD
Dr. Broadstock (@BroadstockMD) is a PGY-3 in Emergency Medicine at the University of Cincinnati and Resident Editor of Ultrasound of the Month.
References:
Dwyer, Kristin H., et al. “Diagnosing Centrally Located Pulmonary Embolisms in the Emergency Department Using Point-of-Care Ultrasound.” The American Journal of Emergency Medicine, vol. 36, no. 7, 2018, pp. 1145–1150., https://doi.org/10.1016/j.ajem.2017.11.033.
Daley, James I., et al. “Increased Sensitivity of Focused Cardiac Ultrasound for Pulmonary Embolism in Emergency Department Patients with Abnormal Vital Signs.” Academic Emergency Medicine, vol. 26, no. 11, 2019, pp. 1211–1220., https://doi.org/10.1111/acem.13774.
Lang, Roberto M., et al. “Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging.” European Heart Journal – Cardiovascular Imaging, vol. 16, no. 3, 2015, pp. 233–271., https://doi.org/10.1093/ehjci/jev014.
Alerhand, Stephen, et al. “What Are the Echocardiographic Findings of Acute Right Ventricular Strain That Suggest Pulmonary Embolism?” Anaesthesia Critical Care & Pain Medicine, vol. 40, no. 2, 26 Mar. 2021, p. 100852., https://doi.org/10.1016/j.accpm.2021.100852.
Ribeiro, Ary, et al. “Echocardiography Doppler in Pulmonary Embolism: Right Ventricular Dysfunction as a Predictor of Mortality Rate.” American Heart Journal, vol. 134, no. 3, 1997, pp. 479–487., https://doi.org/10.1016/s0002-8703(97)70085-1.
Rudski, Lawrence G., et al. “Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography.” Journal of the American Society of Echocardiography, vol. 23, no. 7, 2010, pp. 685–713., https://doi.org/10.1016/j.echo.2010.05.010.
Dresden, Scott, et al. “Right Ventricular Dilatation on Bedside Echocardiography Performed by Emergency Physicians Aids in the Diagnosis of Pulmonary Embolism.” Annals of Emergency Medicine, vol. 63, no. 1, 2014, pp. 16–24., https://doi.org/10.1016/j.annemergmed.2013.08.016.
Rudoni, Raymond R, et al. “Use of Two-Dimensional Echocardiography for the Diagnosis of Pulmonary Embolus.” The Journal of Emergency Medicine, vol. 16, no. 1, 1998, pp. 5–8., https://doi.org/10.1016/s0736-4679(97)00226-6.
McConnell, Michael V, et al. “Regional Right Ventricular Dysfunction Detected by Echocardiography in Acute Pulmonary Embolism.” The American Journal of Cardiology, vol. 78, no. 4, 1996, pp. 469–473., https://doi.org/10.1016/s0002-9149(96)00339-6.
Afonso, Luis, et al. “A Doppler Echocardiographic Pulmonary Flow Marker of Massive or Submassive Acute Pulmonary Embolus.” Journal of the American Society of Echocardiography, vol. 32, no. 7, 2019, pp. 799–806., https://doi.org/10.1016/j.echo.2019.03.004.
Fields, J. M., MD, et al. "Transthoracic Echocardiography for Diagnosing Pulmonary Embolism: A Systematic Review and Meta-Analysis." Journal of the American Society of Echocardiography, vol. 30, no. 7, 2017, pp. 714-723.e4.
Carlsson, Marcus, et al. “The Quantitative Relationship between Longitudinal and Radial Function in Left, Right, and Total Heart Pumping in Humans.” American Journal of Physiology-Heart and Circulatory Physiology, vol. 293, no. 1, 2007, https://doi.org/10.1152/ajpheart.01376.2006.
Pruszczyk, Piotr, et al. “Prognostic Value of Echocardiography in Normotensive Patients with Acute Pulmonary Embolism.” JACC: Cardiovascular Imaging, vol. 7, no. 6, 2014, pp. 553–560., https://doi.org/10.1016/j.jcmg.2013.11.004.
Daley, James, et al. “Emergency Physician Performed Tricuspid Annular Plane Systolic Excursion in the Evaluation of Suspected Pulmonary Embolism.” The American Journal of Emergency Medicine, vol. 35, no. 1, 2017, pp. 106–111., https://doi.org/10.1016/j.ajem.2016.10.018.