Treatment of Anaphylaxis
/If you are reading this, you are in one of two places right now.
You may be sitting comfortably, expanding your knowledge, curious about the latest data for anaphylaxis treatment, and preparing for the next time you may see a patient with anaphylaxis. This resource is for you.
Alternatively, you are actively treating and managing a patient with anaphylaxis, looking for an evidence-based guide to support your clinical decisions in this moment. This resource is also for you.
Feel free to skip down to the “My patient is still hypotensive – help!” section if needed.
Rapid identification and treatment of anaphylaxis is an essential competency for any physician. All medical school graduates, and even much of the lay public, can identify someone becoming acutely ill and/or struggling to breathe after exposure to a known allergen and administer intramuscular epinephrine by following the steps on a package insert.
But then what? And why epinephrine, anyway?
Do any other interventions provide benefit?
How long should a patient be observed before sending them home?
Emergency physicians must understand the evidence (or lack thereof) underlying our treatment of anaphylaxis in the emergency department to deliver the best possible care to these patients.
Does my patient have anaphylaxis?
If your patient fits into one of the three categories below, you may have yourself a diagnosis. [1-5]
The patient’s illness is of acute onset, includes mucocutaneous symptoms and at least one of the following
Respiratory symptoms
Hypotension
End-organ damage due to hypoperfusion
Rapid development of at least two of the following after exposure to a known or likely allergen
Mucocutaneous symptoms
Respiratory symptoms
Hypotension
End-organ damage due to hypoperfusion
Persistent gastrointestinal symptoms
Hypotension after exposure to a known allergen
++ Click for more Background
These criteria were created by the National Institute of Allergy and Infectious Disease (NIAID). They have been prospectively evaluated for use by allergists and emergency physicians and have a 97% sensitivity and 82% specificity for the diagnosis of anaphylaxis. The negative predictive value was 98%, but the positive predictive value was 69%. [6-8] In other words, you probably won’t miss anaphylaxis if you use these criteria, but given the PPV of 69%, patients who meet these criteria may not necessarily have anaphylaxis. Use your clinical judgement appropriately and consider a broad differential diagnosis.
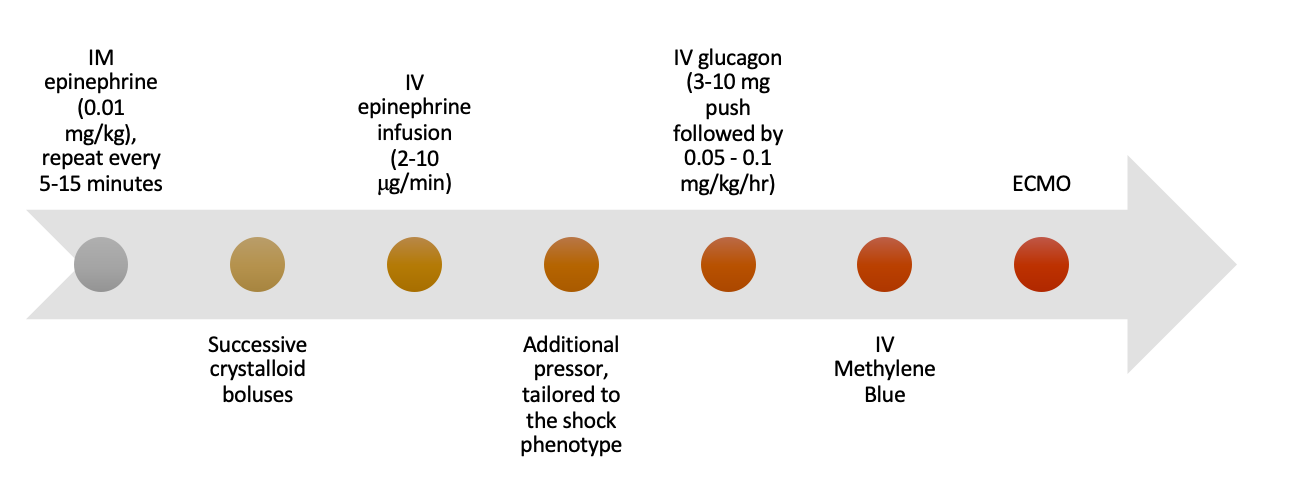
Figure 1 - Treatment Cascade for Anaphylaxis
My patient has anaphylaxis, what is the initial treatment?
Epinephrine, 0.01 mg/kg intramuscularly with a maximum initial dose of 0.5mg, repeated every 5-15 minutes.
Note that the “standard” dose (and the one that is administered by autoinjectors) is 0.3mg every 5-15 minutes, although very few of our adult patients weigh 30kg. You may find yourself using an autoinjector if this is more readily available than a vial of epinephrine, or this may have been used prehospital, so do not be afraid to re-dose the medication if indicated.
Why epinephrine? Click below for the physiology.
First, it’s worth pointing out that there is a Cochrane review on this subject from 2008. [9] Interestingly, the reviewers were unable to find any studies meeting their initial inclusion criteria (randomized, blinded, placebo-controlled) for the review “…to define the true extent of benefits from the administration of adrenaline in anaphylaxis…they may be unethical because prompt treatment with adrenaline is deemed to be critically important for survival in anaphylaxis.” They state they are “unable to make any recommendations based on the findings of this review.” In other words, we’ll never get a “gold-standard” trial for epinephrine’s use in anaphylaxis, so we have to work with what we have.
++ The physiology
Anaphylaxis is initiated by an environmental trigger which causes either an IgE-mediated or non-IgE-mediated inflammatory response. Mast cells and basophils are activated along with myriad other cell lines, the complement cascade kicks off, and there is release of cytokines, histamine, tryptase, nitric oxide, prostaglandins, leukotrienes – all those things you learned about in medical school fire at once. This leads to spasm of smooth muscle, increased vascular permeability, vasodilation, and decreased peripheral vascular resistance (just to name a few of the life-threatening sequelae). [1-3,5,9-11]
Yes, anaphylaxis affects Airway, Breathing, and Circulation all at the same time. And it happens quickly. The median time to cardiopulmonary arrest after exposure to the causative stimulus is between 5-15 minutes if untreated. [6] Fortunately, we have epinephrine. Epinephrine agonizes α1, β1, and β2 receptors. This increases peripheral vascular resistance, thereby reducing peripheral vasodilation and vascular permeability, and aids significantly in bronchodilation. Even more interesting is that epinephrine addresses the root of the problem as well. Agonism of β2 receptors increases intracellular cAMP production in basophils and mast cells, leading to decreased release of their contents. [9,11]
Route of administration? Location of administration? Dose?
Intramuscular. Anterolateral thigh. 0.01mg/kg, repeated every 5-15 minutes.
Maximal plasma concentration occurs more quickly with intramuscular administration than with subcutaneous administration. [5,12] And, although it is frequently cited and repeated, the precise, appropriate dose of epinephrine is not known and there have been no studies performed that elucidate the therapeutic range for epinephrine in anaphylaxis. The dose is empirical and thus varies in the literature and from guideline to guideline. Most guidelines call for 0.3 – 0.5 mg doses every 5-15 minutes as needed. [5,6,13] The anterolateral thigh is used based on several pharmacokinetic studies which indicate faster onset of action and higher mean peak plasma concentration when compared with the deltoid. First-line treatment of anaphylaxis with IV epinephrine is not recommended due to the risks of possible adverse cardiac events, however this may be indicated in refractory anaphylactic shock (discussed more below). [5]
Should I start fluids?
Yes. [2,3,5,6,13]
Use successive IV crystalloid boluses in patients with circulatory collapse from anaphylaxis. This intervention should be performed in parallel with IM epinephrine administration if there is any concern for circulatory collapse.
What labs do I need to order?
You’re obtaining IV access, getting your patient on the monitor, ordering epinephrine IM 0.3-0.5mg, and starting a 1L bolus of LR. What labs do you order?
There are no data to support routine laboratory evaluation of patients with anaphylaxis, as there is no one clear biomarker, but it may help depending on your differential diagnosis. [1,14] You may also want to rule out other causes of hypotension or rash if there was no clear trigger or if the patient has never experienced this before. A 2018 review article in Chest has a robust section on differential diagnosis. [1]
Some newer literature suggests that serum tryptase may be useful in the diagnosis of anaphylaxis, however the performance of tryptase as a biomarker test for anaphylaxis is poor.
++The data for tryptase
Tryptase is released from mast cells during their degranulation in an acute allergic reaction and therefore elevation in this value should theoretically aid in diagnosis. Unfortunately, the sensitivity and specificity of serum tryptase levels are too low and neither the peak nor the delta tryptase levels can effectively be used to aid in the diagnosis of anaphylaxis in the emergency department. Furthermore, there are other confounding factors precluding the use of tryptase as a clinically valuable biomarker for anaphylaxis in the acute setting. As mentioned above, epinephrine use prevents mast cell degranulation at the source and therefore prehospital use of an epinephrine auto-injector could easily cause a falsely low serum tryptase level even in the setting of legitimate anaphylaxis. [14,15] Some guidelines still advocate for ordering the test in the acute setting, with the goal of possibly indicating whether or not the episode in the emergency department was indeed anaphylaxis. [6] In other words, even if you order serum tryptase it won’t help you, but maybe it will help your colleague in the allergy clinic down the line.
My patient has not improved, what should I do next?
Many guidelines, including our own algorithm, advocate for the use of adjunctive treatments in addition to epinephrine for the acute management of anaphylaxis. These include agents such as H1 and H2 antihistamines and glucocorticoids. But what is the evidence for their use?
Antihistamines
With such marginal benefit, and certainly no benefit for the life-threatening symptoms of anaphylaxis, stop to think before you order these medications.
++ The Data for Antihistamines
A Cochrane review, published in 2007, attempted to assess the benefits and harms of H1-antihistamines in the treatment of anaphylaxis. Unfortunately, at that time, there were no randomized, placebo-controlled trials that met their inclusion criteria and thus the authors concluded “…there is no evidence from randomized controlled trials to support the use of H1-antihistamines in the emergency management of anaphylaxis.” [16] With no definitive data, guidelines are mixed on the utility of antihistamines in the treatment of anaphylaxis. Antihistamines make sense intuitively, as we know that they inhibit vasoactive mediator release from basophils and mast cells. [10] However, the data we do have on their use in anaphylaxis seems to indicate that they have a slow onset of action, have negligible effect on blood pressure, and do not relieve bronchospasm or GI symptoms. The only real data supporting their use is from cases of milder allergic reactions where antihistamines (H1 and H2 combined) led to statistically significant decreases in the cutaneous manifestations of anaphylaxis such as flushing, urticaria, or rhinorrhea. [6,12,16-19] Antihistamines have side effects that are not insignificant, especially the anticholinergic properties of tachycardia and sedation. These side effects may cloud the clinical picture and may point you in the wrong direction as you reassess your patient.
Glucocorticoids
Although there is no significant direct evidence of harm associated with their use, there is a paucity of evidence supporting the use of glucocorticoids in the treatment of anaphylaxis.
++ The Data for Glucocorticoids
Once again, we find ourselves with a Cochrane review that yielded no studies meeting inclusion criteria. The authors comment that although there is no convincing evidence for the use of glucocorticoids in the treatment algorithm for anaphylaxis, their use has been increasing in the past two decades. Their empiric use is based on the mechanism of action whereby glucocorticoids down-regulate late-phase eosinophilic inflammatory response thus theoretically reducing the likelihood of biphasic anaphylaxis. Unfortunately, the authors conclude that there is no relevant evidence to support the use of glucocorticoids in the acute treatment of anaphylaxis. [5,20]. [20] Even the newest guidelines continue to recommend treatment with glucocorticoids despite self-reported “very low” rating of the evidence. [5,6]
My patient looks better after initial epinephrine and fluids, now what? Should I give adjunctive treatments? How long do I need to observe my patient in the ED?
In the well-appearing, post-treatment patient, the feared complication is biphasic anaphylaxis—a recurrent reaction or appearance of new symptoms without further exposure to the causative agent.
Various expert panels have made consensus statements and formed clinical practice guidelines for the appropriate observation period after initial resolution of anaphylaxis symptoms, but the jury is still out. Estimates are as high as 20% for the incidence of biphasic anaphylaxis, and observational data suggest that these reactions can occur between 1-72h after the initial symptom onset. The question of how long to observe a patient in the emergency department remains a difficult one. Some guidelines recommend 1-4 hours, some recommend 10 hours, and some recommend a 24-hour observation period. However, current recommendations suggest an individualized approach to observation time based on patient factors. [4,6,12,21-23]
Some studies have attempted to identify risk factors that predispose to a biphasic reaction, with the goal of risk-stratifying patients for various observation periods. The most robust data demonstrate a statistically significant link between severe initial symptoms and the need for more than one dose of IM epinephrine. [5] Other associated factors include increased time between trigger and first epinephrine dose, cutaneous findings, an ingested trigger, and a wide pulse pressure. [5,11,23,24]
More recent studies have called into question the incidence and significance of biphasic anaphylaxis. [11,22,23,25] A recent prospective cohort study of emergency department patients found that the incidence of biphasic anaphylaxis is approximately 7%, and only 5% of those patients had a clinically significant biphasic reaction. A notable finding from this study supports previous data that earlier epinephrine administration is correlated with a decreased likelihood of a biphasic reaction. [23] Additionally, a recent study from Denmark investigated the rate of biphasic anaphylaxis in patients admitted to the intensive care unit. Less than 1% of nearly 15,000 anaphylaxis patients required ICU admission, and, of these, less than 5% had a biphasic reaction. All of these reactions were mild and consisted of cutaneous symptoms only. [25]
What about antihistamines and glucocorticoids?
As discussed above, these have no data to support their efficacy in the acute management of anaphylaxis, particularly related to their delayed onset of action and small role in counteracting the underlying pathophysiology of anaphylaxis. But do they have a role in preventing biphasic anaphylaxis?
No. [5,16,17,21,22,26]
Multiple studies have evaluated this, but none have demonstrated any statistically significant reduction in incidence of biphasic anaphylaxis with the use of these adjunctive therapies. The most contemporary review and practice guidelines state specifically “antihistamines and/or glucocorticoids are not reliable interventions to prevent biphasic anaphylaxis…” and yet many practitioners regularly order them with this goal in mind. As above, there is “very low” quality evidence for the utility of these therapies in anything but reduction of mild, cutaneous symptoms. [5]
My patient is still hypotensive – help! (Anaphylactic shock)
You’ve administered multiple doses of IM epinephrine. You’re on your 3rd bolus of crystalloid. You’ve given antihistamines and glucocorticoids even though you know there’s no good data to support their use. Maybe you’re headed toward intubation for angioedema, waning mental status, or both. Your patient is still hypotensive. You’re getting out the central line kit. What now?
You’re now in the realm of anaphylactic shock. Anaphylactic shock is classically taught to be a distributive shock, but in reality, it is multifactorial. Peripheral vasodilation does indeed cause a distributive shock, but extremely leaky capillaries can also lead to a legitimate hypovolemic shock. Increased heart rate and stroke volumes should be able to partially compensate, but anaphylaxis can also cause myocardial depression and relative bradycardia. Although not completely understood, the massive release of cytokines, prostaglandins, histamine, nitric oxide, and other vasoactive compounds is thought to lead to the negative effects on the myocardium and further complicate the physiology of anaphylactic shock by adding a third, cardiogenic, layer to the shock. [1]
Current recommendations support initiating an IV epinephrine drip between 2-10μg/minute. [3,5,19] If this has been maximally titrated and the patient is still hypotensive, another adjunctive vasopressor may be selected based on the patient’s likely dominant shock characteristic.
If your patient is taking β-blockers and you are concerned this is limiting the action of epinephrine, IV glucagon may be considered. The recommended dosage is a 3-10mg slow IV push followed by an IV infusion of 0.05-0.1mg/kg/hr. Glucagon functions by directly activating adenyl cyclase, producing cAMP without the need for β-agonism, and theoretically producing the same effects of bronchodilation, vasoconstriction, and positive inotropy.[1-3,6]
Animal studies and case reports have indicated a possible role for IV methylene blue in anaphylactic shock refractory to epinephrine. The theoretical proposed mechanism is the inhibition of guanylate cyclase, reduction in cGMP, and prevention of further nitric oxide-mediated vasodilation. [1,13,18]
If you have tried all these interventions and you are unable to achieve shock resolution, ECMO may be considered. In a single case report from 2015, a patient had refractory anaphylactic shock to ioversol contrast during coronary angiography. ECMO was initiated and the patient was discharged 21 days later without any serious complications. [27]
My patient responded well to treatment and is stable for discharge – what do I need to consider?
We’ve already discussed above the data surrounding observation time in the emergency department and potential for biphasic anaphylaxis. Your patient’s symptoms have completely resolved, you have observed them in the ED for a few hours (depending on risk factors associated with their initial presentation), and you have now decided to send your patient home. What do you prescribe? What do you tell them? Where do you refer them?
There is robust evidence to support the prescription of self-injectable epinephrine in patients discharged from the emergency department after treatment for anaphylaxis. Thus, it is recommended that all patients with an anaphylactic trigger that may be encountered in the environment should be prescribed self-injectable epinephrine. In contrast to this, a study from 2008 indicated that only 36% of patients were discharged from the emergency department with a prescription for self-injectable epinephrine and only 31% were referred to an allergist. [28] In more recent years, in both the US and the UK, guidelines have continued to recommend prescription of self-injectable epinephrine, particularly in the form of auto-injectors, as well as allergist referral upon discharge from the emergency department. This is supported by evidence that earlier administration (pre-hospital) is associated with less severe reactions and has a favorable risk-benefit profile. Fortunately, these devices are becoming more and more available and cheaper for patients. The FDA recently approved the first generic epinephrine auto-injector in 2018, expanding access to this life-saving medication. [29] Additionally, referral to an allergist is associated with reduced incidence of future anaphylactic reactions. [30]
Some guidelines recommend prescription of glucocorticoids at discharge, however this has not been shown to decrease biphasic anaphylaxis nor has it been shown to decrease rates of return to the ED. [5] Thus, this is probably not warranted.
As previously discussed, although antihistamines have no data to support their role in managing the acute, life-threatening phase of anaphylaxis, there may be a role in prescription at discharge. There is data to support their use for mild mucocutaneous symptoms and as most biphasic reactions are mild with predominantly mucocutaneous symptoms, antihistamines may provide some benefit in mild symptom management if urticaria or rhinorrhea were to re-emerge after discharge. [1,5,6,12,16,22,26] There has not been a study specifically evaluating this intervention’s impact on patients or ED return visits, however.
Upon discharge, be sure to counsel your patients on appropriate use of the epinephrine autoinjector, allergist follow-up, warning signs for a biphasic reaction, and techniques for avoiding known triggers.
References
Loverde D, Iweala OI, Eginli A, Krishnaswamy G. Anaphylaxis. Chest 2018;153(2):528-543. DOI: https://doi.org/10.1016/j.chest.2017.07.033.
Rowe BH, Grunau B. Allergy and Anaphylaxis. In: Tintinalli JE, Ma OJ, Yealy DM, et al., eds. Tintinalli's Emergency Medicine: A Comprehensive Study Guide, 9e. New York, NY: McGraw-Hill Education; 2020.
Walls RM, Hockberger RS, Gausche-Hill M. Allergy, Hypersensitivity, and Anaphylaxis. Rosen's Emergency Medicine: Concepts and Clinical Practice, 9th Edition: Elsevier; 2018.
Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. Annals of Emergency Medicine 2006;47(4):373-380. DOI: https://doi.org/10.1016/j.annemergmed.2006.01.018.
Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. Journal of Allergy and Clinical Immunology 2020;145(4):1082-1123. DOI: https://doi.org/10.1016/j.jaci.2020.01.017.
Campbell RL, Li JTC, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Annals of Allergy, Asthma & Immunology 2014;113(6):599-608. DOI: https://doi.org/10.1016/j.anai.2014.10.007.
Loprinzi Brauer CE, Motosue MS, Li JT, et al. Prospective Validation of the NIAID/FAAN Criteria for Emergency Department Diagnosis of Anaphylaxis. The Journal of Allergy and Clinical Immunology: In Practice 2016;4(6):1220-1226. DOI: https://doi.org/10.1016/j.jaip.2016.06.003.
Baalmann DV, Hagan JB, Li JTC, Hess EP, Campbell RL. Appropriateness of epinephrine use in ED patients with anaphylaxis. The American Journal of Emergency Medicine 2016;34(2):174-179. DOI: https://doi.org/10.1016/j.ajem.2015.10.003.
Sheikh A, Shehata YA, Brown SGA, Simons FER. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database of Systematic Reviews 2008(4). DOI: https://doi.org/10.1002/14651858.CD006312.pub2.
Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. Journal of Allergy and Clinical Immunology 2017;140(2):335-348. DOI: https://doi.org/10.1016/j.jaci.2017.06.003.
McLean-Tooke APC. Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ 2003;327(7427):1332-1335. DOI: https://dx.doi.org/10.1136/bmj.327.7427.1332.
Dhami S, Panesar SS, Roberts G, et al. Management of anaphylaxis: a systematic review. Allergy 2014;69(2):168-175. DOI: https://doi.org/10.1111/all.12318.
Simons FER, Ebisawa M, Sanchez-Borges M, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organization Journal 2015;8(1):32. DOI: https://doi.org/10.1186/s40413-015-0080-1.
Passia E, Jandus P. Using Baseline and Peak Serum Tryptase Levels to Diagnose Anaphylaxis: a Review. Clinical Reviews in Allergy & Immunology 2020;58(3):366-376. DOI: https://doi.org/10.1007/s12016-020-08777-7.
Buka RJ, Knibb RC, Crossman RJ, et al. Anaphylaxis and Clinical Utility of Real-World Measurement of Acute Serum Tryptase in UK Emergency Departments. The Journal of Allergy and Clinical Immunology: In Practice 2017;5(5):1280-1287.e2. DOI: https://doi.org/10.1016/j.jaip.2017.06.021.
Sheikh A, ten Broek VM, Brown SGA, Simons FER. H1‐antihistamines for the treatment of anaphylaxis with and without shock. Cochrane Database of Systematic Reviews 2007(1). DOI: https://doi.org/10.1002/14651858.CD006160.pub2.
Andreae DA, Andreae MH. Should antihistamines be used to treat anaphylaxis? BMJ 2009;339:b2489. DOI: https://doi.org/10.1136/bmj.b2489.
Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 Update. Journal of Allergy and Clinical Immunology 2010;126(3):477-480.e42. DOI: https://doi.org/10.1016/j.jaci.2010.06.022.
Simons FER, Ardusso LRF, Bilò MB, et al. World Allergy Organization anaphylaxis guidelines: Summary. Journal of Allergy and Clinical Immunology 2011;127(3):587-593.e22. DOI: https://doi.org/10.1016/j.jaci.2011.01.038.
Choo KJL, Simons FER, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database of Systematic Reviews 2012(4). DOI: 10.1002/14651858.CD007596.pub3.
Lee S, Sadosty AT, Campbell RL. Update on biphasic anaphylaxis. Current Opinion in Allergy and Clinical Immunology 2016;16(4):346-351. (Review). DOI: https://doi.org/10.1097/ACI.0000000000000279.
Pourmand A, Robinson C, Syed W, Mazer-Amirshahi M. Biphasic anaphylaxis: A review of the literature and implications for emergency management. The American Journal of Emergency Medicine 2018;36(8):1480-1485. DOI: https://doi.org/10.1016/j.ajem.2018.05.009.
Liu X, Lee S, Lohse CM, Hardy CT, Campbell RL. Biphasic Reactions in Emergency Department Anaphylaxis Patients: A Prospective Cohort Study. The Journal of Allergy and Clinical Immunology: In Practice 2020;8(4):1230-1238. DOI: https://doi.org/10.1016/j.jaip.2019.10.027.
Dribin TE, Sampson HA, Camargo CA, et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. Journal of Allergy and Clinical Immunology 2020. DOI: https://doi.org/10.1016/j.jaci.2020.08.015.
Højlund S, Søe-Jensen P, Perner A, et al. Low Incidence of Biphasic Allergic Reactions in Patients Admitted to Intensive Care after Anaphylaxis. Anesthesiology 2019;130(2):284-291. DOI: https://doi.org/10.1097/aln.0000000000002500.
Kemp SF. The post-anaphylaxis dilemma: How long is long enough to observe a patient after resolution of symptoms? Current Allergy and Asthma Reports 2008;8(1):45-48. DOI: https://doi.org/10.1007/s11882-008-0009-7.
Zhang Z-P, Su X, Liu C-W. Cardiac arrest with anaphylactic shock: a successful resuscitation using extracorporeal membrane oxygenation. The American Journal of Emergency Medicine 2015;33(1):130.e3-130.e4. DOI: https://doi.org/10.1016/j.ajem.2014.06.034.
Campbell RL, Luke A, Weaver AL, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Annals of Allergy, Asthma and Immunology 2008;101(6):631-636. (Article). DOI: https://doi.org/10.1016/S1081-1206(10)60227-X.
FDA approves first generic version of EpiPen. Online: U.S. Food and Drug Administration; 2018.
Ewan P, Brathwaite N, Leech S, et al. BSACI guideline: prescribing an adrenaline auto-injector. Clinical & Experimental Allergy 2016;46(10):1258-1280. DOI: https://doi.org/10.1111/cea.12788.
Written by Tony Fabiano, MD, PGY-1, University of Cincinnati Department of Emergency Medicine
Peer Review and Edited by Jeffery Hill, MD MEd