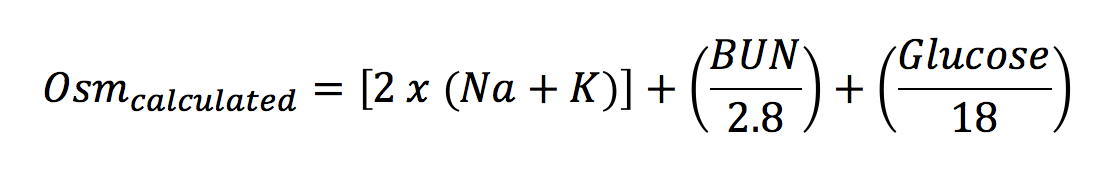How Stuff Works: The Renal Panel Edition
/Have you ever wondered what is actually being measured when you order a renal panel/BMP/serum electrolytes? Well grab your nearest pumpkin spice latte and put your Gilmore Girls Netflix binge on pause because we are about to get a little basic . . . science!
First off what the does lab actually do when you send blood for a renal panel?
Blood for a renal panel/BMP is collected in a serum separator tube (aka a Green Top). These tubes contain a gel that separates blood cells from plasma and clotting agents that speed up blood clotting. In the lab, the tube is then centrifuged and the serum is removed for testing. Serum is the liquid part of whole blood devoid of cells and clotting factors. Serum is then analyzed in a variety of ways to measure different electrolytes and other components of the renal panel. Labs can use a variety of techniques to measure serum components; a few examples are noted below.
- Potentiometry – voltage across an ion-selective electrode with permeable membrane to the ion being measured is compared to a reference electrode. The difference between electrodes results in the serum level.
- K, Na, Cl
- Photometry – the serum component undergoes a chemical reaction to produce a colored chromogen that is then detected by an autoanalyzer
- CO2, Cl, BUN/Creatinine, Mg, Phos
- Enzymatic reaction – component is mixed with a reagent and the byproducts are measured
- Glucose, BUN/Creatinine
Now that we know what happens when we click the Renal Panel lab test button in Epic, let’s look at the individual components of the renal panel.
Sodium (Na)
Sodium is the predominant cation in the ECF; it’s concentration is approximately 140 mEq/L extracellularly and 5 mEq/L intracellularly. ECF volume is governed by sodium, as it is the major osmotically active element in the ECF. Sodium concentration is managed by the addition and removal of water via the thirst reflex and ADH release/inhibition. In the absence of hyperglycemia and renal failure serum sodium is also the major determinant of plasma osmolarity as shown by the following equation below:
Hypernatremia: typically, due to water. Two causes of iatrogenic hypernatremia include patients given large volume of hypertonic saline for resuscitation and infants receiving too much salt in their formula.
Hyponatremia: if serum Na >125 and pt is asymptomatic, do not need to treat emergently. Common etiologies include hyperglycemia and excess water intake. Of note patients that have significant hyperlipidemia/hyperproteinemia can have hypertonic hyponatremia because the water content per volume of plasma is decreased, thus there is less Na per liter of serum.
Potassium (K)
Potassium is the predominant intracellular cation; only 2% of potassium is extracellular. The large intracellular-extracellular gradient is maintained by Na-K ATPase and potassium homeostasis is maintained by the kidney. Many factors can cause potassium to move amongst the cellular realms. Insulin, catecholamines, aldosterone, and alkalemia shift potassium intracellularly and increased osm and academia shift potassium extracellularly.
Hypokalemia: can be due to a loss of total body potassium related to conditions like severe dieting and or/poor intake as seen in conditions like severe alcoholism and anorexia. This can also be due to increased losses from the GI and renal tracts.
Hyperkalemia: can be due to increased total body potassium due to decreased renal excretion as a reflection of intrinsic kidney disease or problems in the renin-angiotensin-aldosterone system. This can also result from academia in the setting of normal total body potassium. Acidosis related to renal failure causes significantly greater hyperkalemia than other organic acidosis.
Chloride
Chloride resides primarily in the ECF and moves around following sodium. Homeostasis is maintained by the kidney.
Hypochloremia: if due to a GI cause, such as vomiting or NG suction, Na will be slightly low and Cl will be markedly low on renal panel. This is because gastric Cl concentration significantly greater than the gastric Na concentration (100 mEq/L vs 20-30 mEq/L). Can also be due to renal losses via diuretic use, salt-wasting conditions, and adrenal insufficiency. If due to extra-renal losses, urine Na and Cl will be low; if due to a renal cause urine Na and Cl will be high.
Hyperchloremia: Often due to free water loss and NaCl administration; can also be seen in salt-water drownings and hypertonic tube feeds if given without free water. Ddx for hyperchloremic metabolic acidosis (Cl>110) includes renal tubular acidosis, primary hyperparathyroidism, and severe diarrhea.
**Fun trivia fact alert**: Bromide intoxication appears as hyperchloremia because it is a chloride equivalent in many measurement techniques. Sx include fever, rash, and AMS
CO2/HCO3
Includes serum bicarbonate (95%) and the available forms of CO2 (carbonic acid and CO2 dissolved in the blood). Homeostasis is maintained by the kidney; in which bicarb follows sodium around. The value given in a BMP is measured via an autoanalyzer through a photometric reaction. On an ABG the bicarb is a calculated value taken from the Henderson-Hasselbalch equation. Bicarb abnormalities reflect changes in acid/base status. Don’t go quaking in your boots – nothing further will be mentioned about acid-base status. But you should know that a bicarb <10 signifies a primary metabolic acidosis; respiratory alkalosis can't drive CO2 that low.
Glucose
Serum glucose concentration is highest in the arterial circulation and decreases as blood becomes more venous. When doing a finger stick, one can warm the fingertip to arterialize the blood and obtain a more accurate FSBS. When drawing venous blood, if the tourniquet is left on >6min the measured glucose can be drop as much as 25. This is because cells consume glucose – for this reason sodium fluoride is added to the serum separator tube to inhibit glycolytic enzymes. Blood can also be rapidly separated or cooled to obtain more accurate results. In the lab glucose is measured via a condensation reaction and analyzed with photometry. Home glucometers measure glucose via an enzymatic reaction in which glucose oxidase and H2O2 are the components actually measured.
Creatinine
Serum creatinine reflects lean muscle mass (approximately 2% stored muscle creatine is converted to creatinine daily. This consistent production of creatinine that is essentially only influenced by changes in muscle mass make it a more reliable estimation of kidney function than BUN. Creatinine is freely filtered at the glomerulus. During renal failure tubular secretion of creatinine increases, but not enough to compensate for the previously free filtration of creatinine ta the glomerulus. Thus at GFR < 50 there is decreased net excretion of creatinine and serum levels rise. Tubular secretion is also affected by ASA, cimetidine, and trimethoprim among many other medications.
Creatinine is measured in a variety of ways. The oldest method is the Jaffe reaction, in which creatinine reacts with alkaline picrate to form a red complex that is then measured by photometry. Unfortunately this method is subject to blood pH and can be affected by many substances including barbiturates, cephalosporins, and glucose. Enzymatic measuring methods tend to be more reliable as they measure ammonia created from the reaction of creatinine and creatinine iminohydrolase.
BUN ("Blood" Urea Nitrogen)
Don’t let the name fool you – BUN is technically the serum urea nitrogen when measured with current techniques. BUN is dependent on many factors including protein intake, endogenous protein catabolism, hydration state, hepatic urea synthesis, and renal urea excretion. BUN can be measured with photometry or enzymatic methods through the measurement of urease breakdown products. Of note BUN only measures the nitrogen component of urea, so the actual serum urea is 2 x BUN. Among many uses BUN is now being used to assess how the nitrogen needs of critically ill patients are being met.
Common Errors Seen in Blood Draw
It happens all the time – you have been waiting 3 hours for a renal panel to finally make a discharge decision on a patient when the lab calls and drops a doozy on you... The sample sent was hemolyzed and the results are essentially useless because you really needed an accurate potassium.
Hemolysis results from breakdown of RBCs causing release of cellular components into plasma. Serum will appear pink when separated from blood component; the darker the serum the more grossly hemolyzed the sample was. Needles size (both too large and too small), tourniquets, alcohol prep, improper syringe draws all contribute to increased rates of hemolysis in blood samples. Thrombocytosis (>600,000) and leukocytosis (>200,000) can also provide results that look like a hemolyzed sample. Potassium is falsely elevated in hemolysis due to the release of intracellular potassium. Phosphate can be mildly elevated but the remaining values of a BMP will be unaffected.
And now for a classic intern problem. You think you are being so helpful by putting in an IV in that swarthy character in room D20, but as you don’t know exactly what labs you want you flush the line and then retreat to the fish bowl to click some boxes. The nurse comes in behind you and pre-emptively draws the blood he/she thinks you will need and send some stuff to the lab. Your BMP comes back and it looks like a hot mess: notably Na 135 Cl 113 K 2.5 and Gluc 50. Your patient is otherwise asymptomatic so what has happened? This is what a BMP looks like when drawn from a flushed line or from a recent NaCl infusion site. It is important to waste some blood before sending labs from these types of IV settings. Classically it has been taught that 3-5 mL of blood should be wasted from flushed sites before blood for tests can be drawn; there are some recent studies that show as little as 1mL of waste can return reliable results.
Written by: Susan Owens, MD PGY-1
Edited, Peer Review, and Posting by: Jeffery Hill, MD MEd
References
- Clinical Methods: The history, Physical, and Laboratory Examinations. 3rd edition. Walker HK, Hall WD, Hurst JW, editors. Boston: Butterworths; 1990. https://www.ncbi.nlm.nih.gov/books/NBK309/
- Determining Optimal Waste Volume From an Intravenous Catheter. Rachel B. Baker, PhD, RN, Suzanne S. Summer, MS, RD, Michelle Lawrence, MS, RN, Amy Shova, BS, Catherine A. McGraw, BA, and Jane Khoury PhD. J Infus Nurs. 2013 Mar; 36(2): 9296. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3587971/
- http://www.surgeryencyclopedia.com/Ce-Fi/Electrolyte-Tests.html