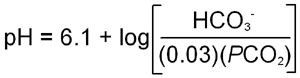Grand Rounds Recap 2/3/16
/This week we had our annual Critical Care Symposium where we invited our own critical care trained faculty and a special guest to have a day chock full of critical care goodness.
Refractory septic shock with Dr. David norton
Dr. David Norton, Assistant Professor of Medicine and Director of the UCMC Medical Intensive Care Unit
Definition of Refractory Shock:
No clear definition exists, but we are generally describing a state of decreased vascular responsiveness despite high vasopressor infusion.
Volume Resuscitation and Fluid Responsiveness
Excessive fluid resuscitation increases ICU LOS, hospital LOS and mortality (Rosenberg AL, et al. J Intensive Care Med 2009;24:35-46, Boyd JH, et al. Crit Care Med 2011;39:259-265)
50% of hemodynamically unstable patients are volume responsive (Boyd JH, et al. Crit Care Med 2011;39:259-265)
The key question: Is there a benefit to be gained from increasing intravascular volume?
Vincent et al. critical care 2011, 15:229
(http://www.ncbi.nlm.nih.gov/pubmed/21884645)
Answering this question is complex...
Are we measuring what we think we're measuring?
Does it mean what we think it means?
Most of the blood in the body is in the venous system:
- Systemic Venous System: 64%
- Systemic Arterial System: 13%
- Capillaries: 7%
- Pulmonary Circuit: 9%
- Heart: 7
This means that venous resistance is a major player in cardiac output. The difference between upstream and downstream is very small which means that small changes in resistance are important but difficult to predict
- As preload goes up, CO goes up per the Starling curve
- CVP is not a consistent indicator of either CO or fluid responsiveness
- There is no consistent relationship between right atrial pressure and cardiac output
- CVP correlates best with volume responsiveness at extremes of intravascular volume (where measuring such a volume is likely least helpful clinically)
- Respiratory variation in CVP does actually predict volume responsiveness
Vasopressors work by increasing venous vascular tone and increasing venous return to the heart. The arterial effects are actually deleterious and decrease CO.
How do we measure "fluid responsiveness"?
Pulse-Contour Analysis (PCA) Related Technologies
There are, essentially, three types of PCA systems:
- PCA systems that require indicator dilution for calibration
- Transpulmonary thermodilution
- LiDCO
- PCA systems that require stored data for calibration
- Flotrac/Vigileo
- PCA systems that require neither
NiCOM
Uses a proprietary algorithm utilizing impedence as a method for calculating CI/CO
IVC Variability
Probably a good way to predict volume responsiveness but has some operator variability based on where you measure and what you use as a cutoff value
“Most refractory shock is actually inadequate volume resuscitation. Friends don’t let friends remain under-resuscitated.”
Other Therapies for Refractory Shock
There are various proposed mechanisms of vascular hyporespnsiveness in sepsis. NOS (cNOS, iNOS, mtNOS) is upregulated in sepsis and NOS inhibitors are, so far, not shown to have good results
Methylene blue for refractory septic shock works to prevent this nitric oxide mediated vasoplegia
Other Cutting-Edge Therapies Discussed
- Angiotensin II
- Clonidine
- Prostacyclin inhibitors
- Vasopressin and Selepressin
- Blood Purification (High-Volume Hemofiltration, Therapeutic Plasma Exchange and Hemadsorption)
- ECMO
Critical thinking in critical care with Dr. Erin McDonough
Dr. McDonough started her lecture with three case presentations of critically ill patients who present to the ED...
Case #1:
Case #1 Laboratory Values
- 74 y/o F PMH DM p/w AMS
- VS: BP 70/30, P 122, T 100.8F, RR 22, SpO2 100% on RA
- Nonfocal examination
- AP CXR normal, NCHCT normal
- Urinalysis: 40 WBC, 5 RBC, small LE, 5 sqamous cells
- 2L NS bolus given
- Repeat VS: BP 80/40, P 125, RR 22, SpO2 100%
- Diagnosed with "UTI with Sepsis"
- Antibiotics given. Admitted to MICU.
Case #2:
Case #2 laboratory values
- 68 y/o M PMH ESRD on HD, EF 20% p/w AMS
- VS: BP 88/50, P 92, T 99F, RR 22, SpO2 97% on 2L NC
- Arrives from HD center after 3/4th of HD completed
- Sleepy, opens eyes to voice, answers questions but confused
- Course ronchi diffusely, 3+ LE edema
- AP CXR nondiagnostic with pulmonary edema
- NCHCT normal
- 1L NS bolus given
- Repeat VS: BP 84/48, P 98, RR 24, SpO2 92% on 4L NC
- Diagnosed with "CHF exacerbation"
- Started on norepinephrine infusion. Admitted to MICU.
Case #3
Case #3 laboratory values
- 54 y/o F PMH COPD p/w acute respiratory failure
- VS: BP 105/75, P 96, T 100.5F, RR 38, SpO2 94% on 100% NRB
- In distress, tachypneic, distant breath sounds
- Given beta-agonists, solumedrol and started on bilevel NIPPV
- Intubated for persistent respiratory failure
- AP CXR with RLL "atelectasis versus pneumonia"
- Repeat VS: BP 100/65, P 95, RR 16, SpO2 94% on 100% FiO2
- HCAP antibiotics given
- Diagnosed with "Healthcare Associated Pneumonia". Admitted to MICU.
trying to fit a square peg into a round hole
What do each of these cases have in common?
In all of these cases we were faced with a clinical scenario that almost fit a certain diagnosis, but not quite.
In each case, the patient was given a diagnosis that explained much but not all of the clinical picture, some information was weighed inappropriately over other information, or we tried to make the data fit the diagnosis rather than vice versa...
We often use mental shortcuts and play the odds to come to a diagnosis with limited information. We do this with both high acuity and low acuity patients, though with the lower acuity patients it more rarely results in a bad outcome.
For high acuity patients, going all-in on a diagnosis that sort of fits is a huge gamble because we may miss life-saving interventions which are time-sensitive before we realize we were wrong.
“The emergency physician must often make complicated clinical decisions with limited information while faced with a multitude of competing demands and distractions.”
Unfortunately, we rarely have the luxury to sit back and wax philosophical about our patient's presentations and develop extensive, CPC-worthy differentials. We work in an environment that is high risk for cognitive errors...
Cognitive Errors
According to Kovacs and Croskerry, cognitive errors can be grouped into four main types:
- Faulty hypothesis generation
- This is failure to consider a diagnosis. If you don't think of it then you can't diagnose it.
- More likely to occur with atypical presentations and uncommon diseases
- Faulty context formulation
- These are mistakes made based on lack of knowledge, inexperience or anything that results in a narrow view of your patient
- There is an over-reliance on mental shortcuts or "heuristics"
- Heuristics are mental shortcuts or associations that can be fraught with peril
- These are often called "pattern recognition"
- Sutton's Slip is an error that occurs when one only considers the obvious (i.e. when obvious orthopedic trauma causes you to miss the intra-abdominal injury)
- Faulty information gathering and processing
- This can be faulty estimation of or over-reliance on disease prevalence
- In some specialties, this is favoring rare or "zebra" diagnoses over common things
- In EM, this is usually the opposite. We under consider zebras and favor "playing the odds"
- Anchoring is a failure to consider alternative diagnoses once a label has been given
- Vertical-line failure is sticking with a diagnosis, even when diagnostic testing or physical exam isn't confirmatory and results from a failure to consider alternative diagnoses in the face of this evidence
- This can be faulty estimation of or over-reliance on disease prevalence
- Faulty verification
- Failure to recognize a "poor fit" and exclude other possible diagnoses
- "Zebra retreat" - not pursuing a diagnosis because the workup is difficult and the disease process is rare
- Premature closure
How do we protect ourselves from these errors?
Metacognition:
"One of the distinguishing hallmarks of adult human intelligence."
- Awareness of the learning process itself
- What does it take to memorize something vs. understand the concepts vs. both?
- Recognition of limits of memory
- Knowing when memory aids or coping strategies are needed
- Ability to appreciate perspective
- Step back from the immediate problem and get the big picture, expand the differential, find the cause behind the clinical picture - allows us to recognize atypical presentations or when data does not fit together
- Capacity for self-critique
- Willingness to reexamine a diagnosis in light of new information or input from other team members, recognizing that your decision making is faulty, such as at the end of a 12-hour shift
- Ability to select strategies
- Choose a decision-making strategy that fits a given scenario. Pattern recognition vs. worst-first vs. common things being common vs. classic pathophysiology
Back to the cases...
Case #1: Abdominal exam revealed significant tenderness. CT abdomen/pelvis showed ischemic bowel and went to OR with surgery.
- Faulty hypothesis generation
- Failure to consider ischemic bowel
- Faulty information gathering and processing
- UA interpretation: 40 WBC - not consistent with severe sepsis/septic shock
- Lactate 7.2
- Vertical-line failure
- Faulty verification
- Making the data fit the diagnosis
Case #2: Blood cultures grew out Gram negative rods. Pitting edema was actually from distributive shock and not CHF exacerbation.
- Faulty context formulation
- Over-valuing CHF vs. ESRD on HD
- Faulty information gathering and processing
- Faulty estimation of disease prevalence
- Anchoring on EF and failing to give more fluid to resuscitate septic shock
- LE edema as a "red-herring"
- Troponin/BNP are not always diagnostic or as useful in ESRD
Case #3: CTPA showed massive PE
- Faulty hypothesis generation
- PE was not initially considered
- Faulty information gathering and processing
- Lung exam lead to COPD diagnosis
- pCO2 of 53 not consistent with this severe of a COPD exacerbation
- High FiO2 requirement despite a not very impressive chest x-ray ("atelectasis vs. pneumonia")
- Vertical-line failure
- Faulty verification
- Making the data fit the diagnosis
- "Zebra retreat"
- Premature closure
Take-Home Points:
- Potential for error is everywhere
- Critically ill patients often require multiple minutes of thought
- If things don't fit then don't force it. Stop, go back to your differential and ask "what are we missing"
- What we set in motion in the ED, our diagnostic testing, therapeutic interventions and diagnoses, carries momentum into the patient's hospital course.
Interpretation of acid/base physiology by dr. william knight
Dr. Knight started with a case to discuss his approach to acid/base physiology...
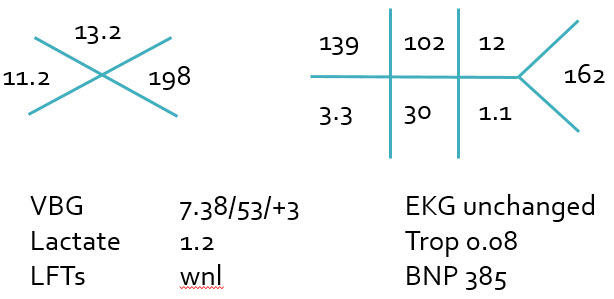
33 y/o F triaged to SRU2
- CC: "I can't breathe"
- VS: T 37.0, BP 137/96, P 137, RR 35, SpO2 80% on 2L NC
- Has difficulty moving from wheelchair to stretcher. Is agitated and is hard to get on the monitor and get IV access. She is muttering incoherently
- HPI: She was off of work today, but showed up at her job as a police dispatcher and was acting strangely. 911 was called
- No PMH, takes no medications, no illicit substance history and no known drug allergies
- She is not cooperative with neuro exam, but does not have any clear deficits. The remainder of her exam is nonfocal.
- EKG shows sinus tachycardia and CXR is normal. NCHCT normal.
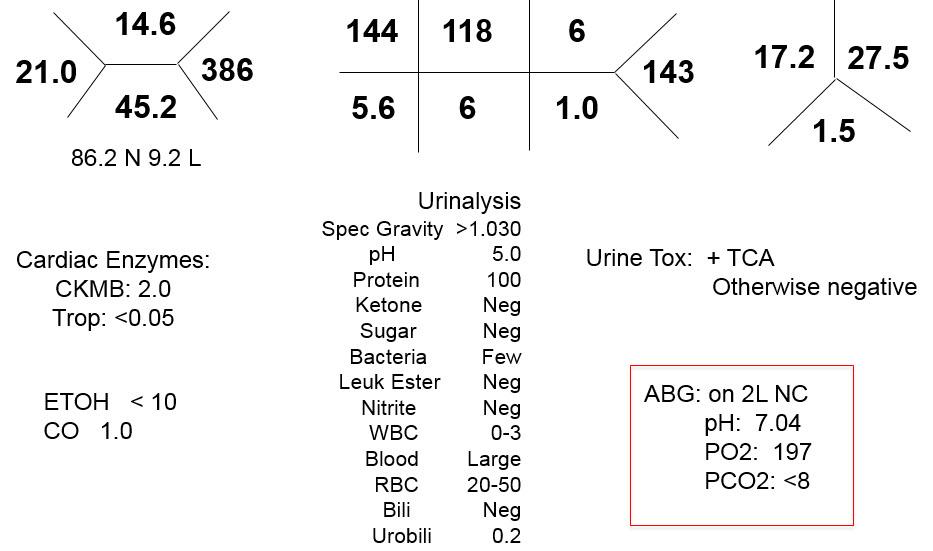
- She has a declining mental status and is intubated for airway protection. The following labs are obtained prior to intubation:
How do we begin to approach/interpret her metabolic abnormalities?
Break it down and keep it simple...
- Acidotic or Alkalotic?
- Gap or Non-Gap?
- Is there compensation? Are there additional processes?
- What is the delta gap?
- What is the osmolar gap?
- If the rule of 15 does not apply, should be suspicious of a second process.
Acidosis or Alkalosis:
- Look at pH first
- 7.04
- Acidotic
Anion Gap
- Na - (HCO3 + Cl) = Anion Gap
- Normal is 5-12
- In our patient, 144- (6 + 118) = 20
Compensation or Additional Processes
the modified henderson-hasselback equation for a bicarbonate buffering system
- This can calculated using the Henderson-Hasselback equation...
- Or, you can use the "Rule of 15" (and then tell people you used Henderson-Hasselback)
- (HCO3 + 15) should = pCO2 and pH (last 2 digits)
- Allows prediction of pCO2 and pH based on knowing the HCO3
- Only works when HCO3 is 10 or greater
- As HCO3 falls below 10 and approaches 5, the pCO2 should go to 15
- For our patient, the HCO3 is 6, but the pCO2 is lower than expected (<8 when it should be 15) indicating a concomitant respiratory alkalosis
Delta-Delta or Delta Gap
- Based on the 1:1 concept that the increase int he anion gap should be equal to the fall in bicarbonate
- Assume normal HCO3 = 24
- Assume normal anion gap = 12
- In our patient:
- dAG = 20-12 = 8
- dHCO3 = 24-6 = 18
- The dHCO3 is "too high" or HCO3 is "too low" to be completely explained by the anion gap
- Indicates a concomitant secondary non-gap metabolic acidosis
Osmolar Gap
- Osmolality = 2*Na + glucose/18 + BUN/2.8 + EtOH/4
- But this number is only truly useful if you have a baseline and calculated Osm to compare to
Our patient had...
- Anion Gap Metabolic Acidosis
- Primary Respiratory Alkalosis
- Non-gap Metabolic Acidosis
When you seen an anion gap metabolic acidosis, think... MUDPILES
- Methanol
- Profound acidosis
- Engorged redina, papilledema
- Abdominal pain
- Uremia
- BUN >60; usually >100
- Creatinine >5; usually >10
- DKA and AKA
- Glucose and history
- Variable glucose
- In AKA, acidosis cured with volume and glucose
- Diagnosis of exclusion
- Paraldehyde
- No longer readily available in the US
- INH
- GABA inhibitor
- Refractory seizures
- TB history (or someone in the home with TB history)
- Iron
- Children, pregnant or post-partum women
- GI bleed
- Abdominal plain film occasionally useful
- Lactic Acidosis
- Multifactorial (seizure, hypotension, sepsis, CO poisoning, CN poisoning, etc...)
- Very high anion gaps
- Type A - poor perfusion or oxygenation
- Type B- classically when evidence of poor perfusion or oxygenation is absent
- Ethylene Glycol
- Seizures, arrhythmias, renal failure
- Initial presentation can seem intoxicated
- Oxalate crystals in urine
- Can also build up lactate
- Antifreeze, windshield washer fluid
- Salicylates
- Primary respiratory alkalosis plus anion gap metabolic acidosis
- Can be mistaken for DKA or hypoglycemia
- Seizures, AMS, GI bleeding
- It's in all sorts of stuff including oil of wintergreen, pepto bismol, BenGay, etc...
Ultimately, this patient was determined to have an ethylene glycol ingestion confirmed by a toxic alcohol panel.
Critical Care Literature Update with Dr. CHristopher Zammit
Sepsis, EGDT and Lactate
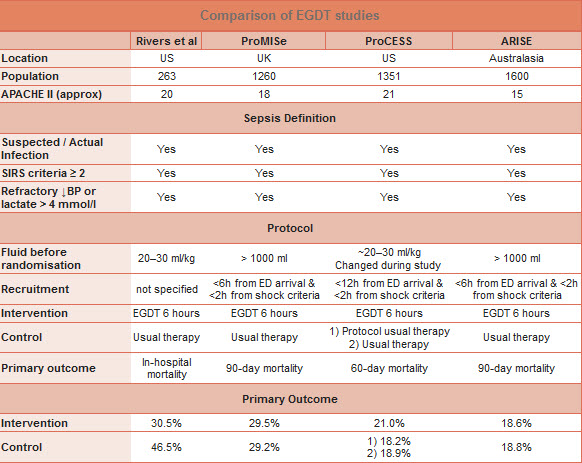
- New studies here are the PROCESS, PROMISE and ARISE trials
- These trials essentially indicate that EGDT is equivalent to "usual therapy"
- What the heck was EGDT and the Rivers trial?
- EGDT per the Rivers trial looked at optimizing central venous oxygenation versus usual care
- The goal of the study was to determine of optimizing oxygen delivery improved outcomes
- The absolute difference in mortality was 16% improvement with a NNT = 6
- If you dig into the data, most of the effect was probably due to transfusion of blood as this is what most of the intervention group got compared to the usual care group. Only a small proportion of patients were started on vasopressors
- This table from The Bottom Line summarizes the results of the ProCESS, ProMISE and ARISE trials compared to Rivers' EGDT:
- Let's talk about lactate:
- Lactic Acidosis in Sepsis: It's Not All Anaerobic (CHEST 2016; 149(1):252-261)
- Anything that increases the conversion of glucose to pyruvate may increase lactate because it overwhelms the enzymes required to convert it to ATP
- There is likely mitochondrial dysfunction, enzymatic dysfunction and/or increase of NADH in sepsis that contributes to rising lactate but is not directly a result of anaerobic metabolism and may not improve with increased oxygen delivery
- Sepsis Bottom Lines:
- Use the "screening" lactate liberally
- Give fluids (minimum 20ml/kg)
- Identify source and get appropriate anti-microbials on board ASAP
- Restore MAP with pressors if needed
- If lactate high, check again in 2 hous. If not clearing appropriately, consider other causes...
- One last sepsis pearl: We know CVP is "dead", but how do we know when fluid will help our patient?
- Passive Leg Raise!
- Not as confounded by cardiac rhythm or respiratory status
- Does look pretty silly when you're actually doing it
- Volume responsiveness definition is >/= 9% increase in CO/CI/SV/SVI with PLR (Note: NOT an increase in BP)
- Passive Leg Raise!
ARDS
- ARDS is defined by the P/F ratio
- ARDS definiton we use clinically is the Berlin definition
- The ARDSNet trial is the data on which we base our "lung protective strategy" of low tidal volume ventilation
- This year in NEJM, there was published Driving Pressure and Survival in the Acute Respiratory Distress Syndrome which indicated that driving pressure was most strongly associated with mortality outcomes and that neither Tv nor PEEP was independently associated with survival.
- Bottom Line: Driving pressure may be a target for therapy, but prospective trials are needed
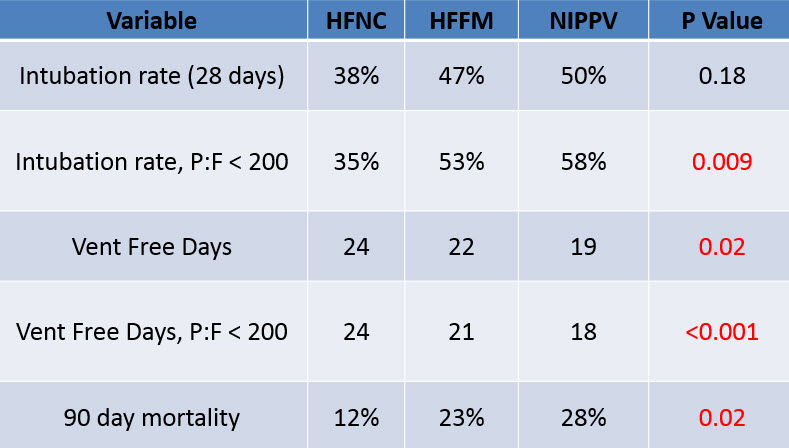
HFNC in Acute Hypoxemic Respiratory Failure
- Also this year in NEJM, there is the High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure study
- In our hospital, we call this Optiflow and can dial our system up to 90L of flow and blend in air and oxygen to target an FiO2
- Incusion criteria:
- P:F < 300 on 10L O2 or more
- RR>25
- PaCO2 <45
- No chronic respiratory disease
- Exclusion Criteria
- Acute, Decompensated HF
- Asthma or COPD Exacerbation
- HCS < 13
- Hemodynamic Instability/Vasopressor Requirement
- Severe neutropenia
- DNI advance-directive
- Contraindications to NIPPV
- Incusion criteria:
- Bottom Line: HFNC appears to be a reasonable first option for patients with AHRF secondary to pneumonia, not requiring intubation and without chronic respiratory disease
Oxygen for Acute MI
- The Air Versus Oxygen in ST-Segment Elevation Myocardial Infarction trial was a randomized-controlled, prehospital study of STEMI patients with 8L NC versus room air
- Bottom Line: Data are lacking to support providing supplemental oxygen to norm-oxemic ACS patients and there is data suggesting there may be harm
Refractory hypoxemia and ventilator management by Dr. Jordan bonomo
The Current State of Mechanical Ventilation in the ED
- Based on a study done in CHEST (CHEST 2015; 148(2):365-374)
- 4 academic ED's included (one was ours)
- Up to 1 in 5 of all non-elective ED intubations will be dead at 28 days
- 7% of patients had ARDS in the ED
- Only 55.7% received lung-protective tidal volumes (LTVV)
- 11.4% got >10 ml/kg of predicted body weight
- Only 32% had tidal volumes changed on arrival to ICU
- We MUST do a better job of LTVV. 6-8 mL/kg of PWB is absolutely required. Have your RT calculate it based on height and weight - it's their job and passion
Lung Protective Ventilation, PEEP and Lung Recruitment
- Open lung ventilation– optimizing peep in setting of low Vt ventilation to maintain alveolar recruitment and decrease alveolar collapse ; that is always our goal in mechanical ventilation
- PEEP decreases de-recruitment– 8 is the new 5
- Recruitment and PEEP are inextricably linked
- PEEP has three primary objectives
- Adequate gas exchange
- Lung protection
- Maintenance of hemodynamics
- If you keep the pressure delta between the Pplatea and the PEEP reasonable, you will limit lung strain
- Setting PEEP (most important part of the talk)
- The PEEP you need to keep the lungs recruited is LOWER than the PEEP you need to initially recruit the lungs
- Full recruitment requires high pressures (NOT volumes)
- IF the pressure support is fixed in relation to PEEP, Vt does not change when PEEP is raised (assuming no hemodynamic obstruction)
- PEEP increase for recruitment ONLY works after recruitment maneuvers (Paw x t)
- PEEP increase for oxygenation works at any time and is NOT recruitment based
- 5 Keys to Setting PEEP
- Lung opening is a continuous function of volume
- Full recruitment requires high pressures
- Recruitment is time dependent
- Recruitment is transient if PEEP is unchanged after
- Most collapse is averted by modest PEEP
- Repetitive maneuvers are better tolerated
- "the first one hurts" when it comes to recruitment maneuvers with respect to hemodynamics
- Good lung recruitment can even prevent ECMO or proning, it is often just not well done
- Methods of Recruiting
- High CPAP
- 40 cmH2O x 40 seconds ("40 for 40")
- This is the easiest and most likely recruitment maneuver to be of benefit in the ED
- Patient considered a responder if there is a >50% increase in PaO2/FiO2
- Responders have an average of ~175% increase
- PEEP should be set +2 above Pflex after recruitment
- Ensure you take your ETT cuff to >50 cm H2O before initiating the maneuver
- Intermittent, stepwise high PEEP with fixed pressure control
- Fixed pressure support (targeting volume of 6mL/kg)
- Increase PEEP in fixed time steps (30s-2min)
- PEEP: 25, 30, 35, 40, 45
- Decrease PEEP by steps of 5 cmH2O until 2-4 cm above starting PEEP
- Use EXTREME caution when doing this in the ED
- High CPAP
- Recruitment- there is a cost
- Extreme prejudice and caution should be used when deciding to use aggressive recruitment measures in the ED
- Hemodynamically unstable patients do NOT warrant recruitment
- Stable patients may benefit
- You may need to prep for hypotension with pressors, including push doses
- Transpulmonary Pressure
- P(transpulmonary) = P(airway) - P (pleural)
- It is NOT true that this must always be positive or our lungs would collapse like we were taught in physiology
- P(esophageal) can be used as a marker for measuring P (pleural) and can be used to guide PEEP
- Mechanical Ventilation Guided by Esophageal Pressure in Acute Lung Injury
- This study showed that the PEEP that is needed is often much higher than we are used to
- PEEP was set to achieve a P (transpulmonary) of 0-10 cm H2O at end-expiration
- Tidal volume was limited to keep P (transpulmonary) at end inspiration < 25 cm H2O
- PaO2:FiO2 ratio was 88 mmHg higher in the P(esophageal) group than the ARDSnet group
- PEEP of 10-14 in resuscitated patients are not unreasonable at all as long as 6 ml/kg is the tidal volume target
- Proning
- The PROSEVA study
- Looked at ARDS patients <36 hrs from intubation and the primary outcome was mortality at 28 days
- Prone: 16.0%
- Supine: 32.8%
- Bottom Line: Don't be afraid to prone in the ED if you must to keep the patient from dying
- ECMO
- CESAR trial
- ECMO referral was the actual intervention
- Absolute risk reduction: 16%
- NNT 6.2
Advanced lung ultrasound with Dr. Jordan Bonomo
Advanced lung ultrasound is very useful as a bedside diagnostic tool and is generally considered easier to acquire and interpret the images than other advanced ultrasound like cardiac
A panel of international experts have published the following Level A recommendations with respect to bedside lung ultrasound (Intensive Care Med (2012) 38:577-591)
- Lung US more accurately rules out PTX vs. CXR
- Lung US differentiates consolidations due to PE, PNA or atelectasis
- In mechanically ventilated patients, lung US more accurately distinguishes between types of consolidation than CXR
- B lines are proportional to the degree of congestion in heart failure
- Pleural effusions with internal echoes are exudates or hemothorax
- Lung US is as accurate as chest CT for detection of pleural effusions
- Lung US - The Fundamentals
- Basics
- Phased array for edema/effusion/pneumothorax
- 3.5-5.5 Hz
- Linear array for pneumothorax/rib fractures
- 7.5 Hz
- Phased array for edema/effusion/pneumothorax
- Goal Directed Scans
- Pleural Effusion (PLEF)
- Pneumothorax (PTX)
- Edema/Aeration/Recruitment
- Basics
- Orientation
- Indicator cephalad (dot to the head)
- Longitudinal axis
- 3 scanning axes
- Mid-clavicular
- Anterior axillary
- Posterior axillary
- Pleural Effusion (PLEF)
- 3 cardinal features
- An echo free space
- Typical anatomic boundaries
- Diaphragm
- Interior chest wall
- Lung
- Typical dynamic changes
- Beware of the diaphragm - intra-diaphragmatic device insertion is often lethal
- Curtain sign is lung that slides into the effusion during the respiratory cycle
- 3 cardinal features
- Pneumothorax
- You are looking for lung sliding
- On M-mode, you are looking for the "sea shore sign" or "stratosphere sign"
- "Stratosphere sign" is due to the absence of parenchymal lung movements and appears as parallel horizontal lines
- Seeing a lung point is 100% specific for pneumothorax
- Lung ultrasound in trauma has been shown to be much more sensitive and almost as specific as a supine CXR (Critical Care 2007, 11:205)
- What about those "B" lines?
- B-lines
- discrete laser-like vertical hyperechoic reverberation artifacts that arise from the pleural line (previously described as "comet tails")
- extend to the bottom of the screen without fading
- move synchronously with lung sliding
- The anatomic and physical basis of B-lines is not known with certainty at this time though they are thought to be reverberation artifacts that originate from the interlobular septa thickened by fluid
- The term "sliding" (rather than "gliding") should be used in description of pleural movement
- What "B" lines tell us
- Multiple B-lines are the sonographic sign of lung interstitial syndrome
- Pulmonary edema of various causes
- Interstitial pneumonia or pneumonitis
- Diffuse parenchymal lung disease (pulmonary fibrosis)
- A positive region is defined by the presence of three or more B-lines in a longitudinal plane between two ribs
- "B" to "A" conversion can indicate successful lung recruitment (Am J Respir Crit Care Med. 2011 Feb 1;183(3):341-7)
- Multiple B-lines are the sonographic sign of lung interstitial syndrome
- B-lines