Air Care Series: No Lung No Problem - VV ECMO
/Background:
Acute respiratory distress syndrome (ARDS) is a complex disease process for which much time and literature is devoted. The premise of incorporating Venous Venous Extracorporeal membrane oxygenation (VV ECMO) as a strategy for the most severe of ARDS patients is to allow for “lung rest” on minimal ventilator settings to minimize iatrogenic complications of further lung injury through high pressure and oxygen settings. VV ECMO is now commonly considered in patients with ARDS who have been paralyzed, proned, and still cannot be oxygenated or ventilated due to high pressures and poor gas exchange.
In addition to ARDS, there are other disease processes that benefit from VV ECMO. Note that all of these processes are due to a primary RESPIRATORY etiology in a patient with preserved circulatory support. Tracheal injury with inability to oxygenate or ventilate, severe pneumonia, pulmonary contusions, bronchopleural fistula, COPD and asthma are just a few examples where VV ECMO may be considered in the most severe cases. (1)
Placing a patient on VV ECMO allows for weaning the ventilator to prevent further iatrogenic injury to the lungs and allow any healing to occur.
Indications and Contraindications:
Several scoring rules have emerged in assessing a patient for VV ECMO but the overall concepts are:
High risk of death despite optimization of medical treatment
Diagnosis of possible reversible disease
Absence of contraindications
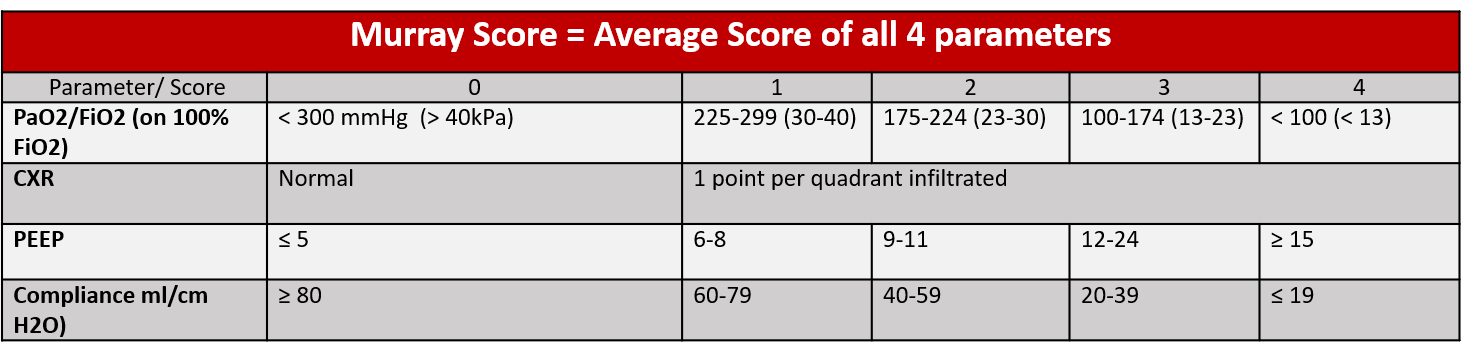
Figure 1: ESLO (1), ECMOnet(2), CESAR (3), and EOLIA (4) criteria for initiation of ECMO are displayed in figure 1.
The Murray Score (an average of four parameters) evaluates injury severity in patients with lung injury
VV ECMO should be implemented in patients whose predicted hypoxemia related morbidity and mortality outweigh the complication risks of VV ECMO. There is debate on when exactly to initiate VV ECMO, with multiple different proposed criteria displayed in Figure 1. At the present point in time, VV ECMO is considered as a bridge to recovery and not destination therapy though there are some patients awaiting lung transplant on VV ECMO for an extended period of time. These patients are often extubated, awake, alert, and ambulatory with dual lumen cannulas.
Though we will not further specifically cover refractory hypercarbia, this is an indication for VV ECMO and also for extracorporeal CO2 removal (ECCO2R). ECCO2R does not facilitate oxygenation but could be of particular use for patients with COPD, asthma, or patients with high plateau pressures.
Adapted from the ELSO Red Book (1)
VV ECMO Configuration:
As the name states, blood is taken from the venous system utilizing negative pressure through the “drainage” cannula, taken through the oxygenator, and returned through the “return” cannula also in the venous system. VV ECMO provides no circulatory support. It does allow for removal of carbon dioxide and return of oxygenated blood. Some patients who are on vasopressors may still benefit from VV ECMO as opposed to VA ECMO (stay tuned for a future podcast) if the presumed cause of the hemodynamic instability is from hypoxia or hypercarbia leading to acidosis.
Smaller, transport circuits have made the transport of ECMO feasible. Some patients will already be cannulated at the referring facility, while other patients will be pending cannulation prior to transport and this will be assisted by the transport team.
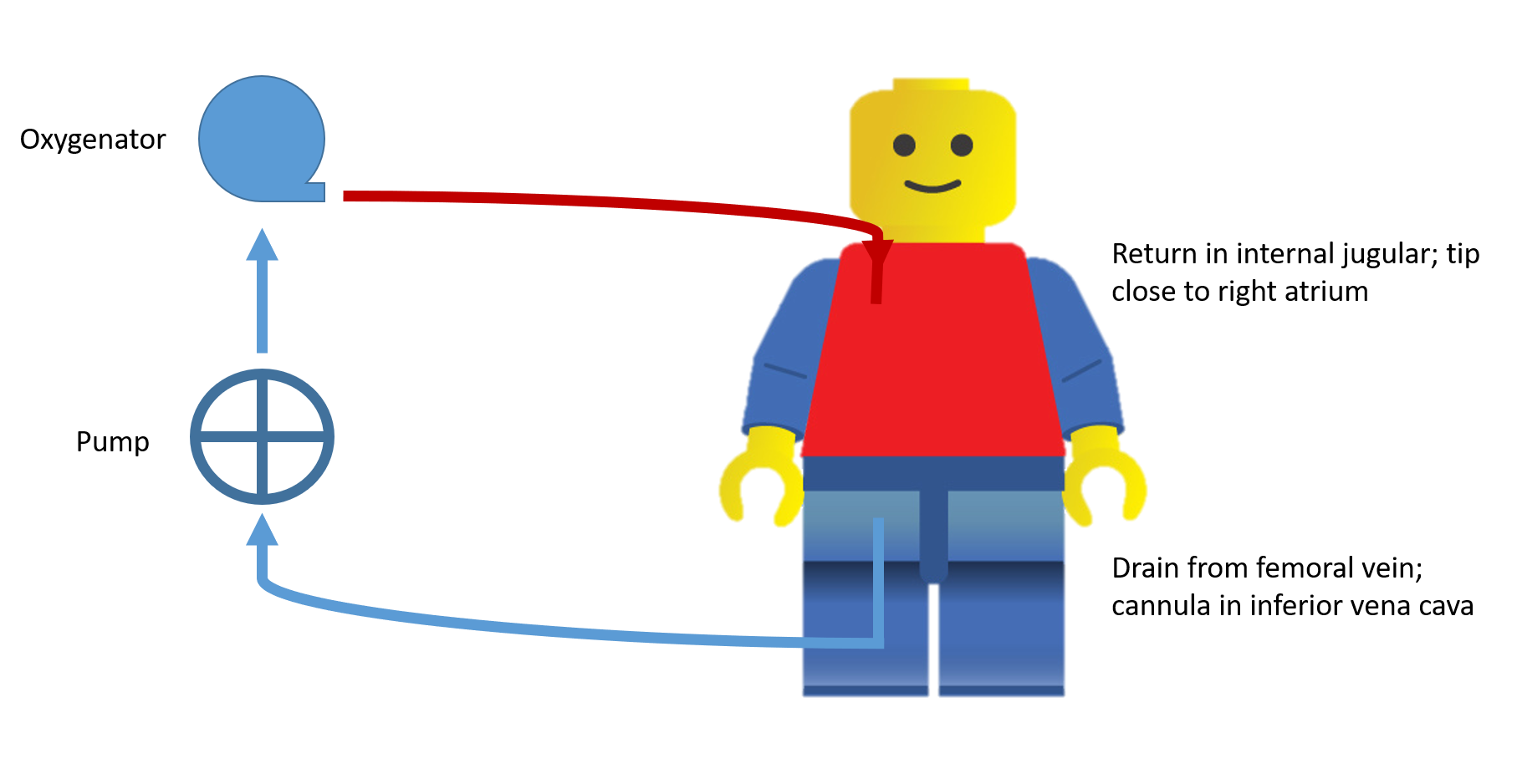
Let’s take a closer look at the circuit. Start at the drainage cannula and trace the blood flow.
Via the motor, negative pressure allows for blood removal from the body via the drainage cannula. This is deoxygenated blood.
Blood passes though the motor to the oxygenator.
The blood passes from the pre to post oxygenator side and this diffusion gradient allows carbon dioxide to be removed and oxygen to diffuse into the blood.
On the post oxygenator side, blood is returned to the body via the return cannula.
VV ECMO Circuit Schematic
There are several parameters that need to be considered to effect oxygen delivery, carbon dioxide removal, and flow of blood (1):
Cannula size - the size of the cannulas will affect the removal and return of blood. Larger cannulas (specifically the drainage cannula) will allow for more blood flowing through the circuit and returned to the patient. Patients with larger body surface area will require higher flows. Some patients may even require a second drainage cannula to allow for enough flow to oxygenate the body.
Sweep - Sweep gas flow is a gas flow in liters per minute through the membrane oxygenator. A higher sweep (0-10) will clear CO2 more rapidly.
Blood Flow Rate - Oxygen delivery depends on blood flow rate. The FdO2 is set on the circuit and can be weaned down as patient pulmonary function improves, however, during initial cannulation is usually set at 100%. The goal PaO2 is >55. Increasing flow (L/min) by increasing the RPMs will improve oxygen delivery, however, increasing the RPMs too much can also lead to chugging (discussed later).
Pressures - Negative pressures should be monitored on the circuit. Negative pressures that are too high (more negative) can lead to suction events, low flows and chugging. Lower flows will decrease oxygenation.
In summary, on initial cannulation and initial transport management, the FdO2 can be set to 100%, the sweep can be titrated for pCO2, and negative pressures should be constantly monitored. Cannula size, location, and type should also be noted.
Cannulation for Adults with Respiratory Failure:
As a transport provider, you may find yourself involved with a cannulation at an outside hospital or may pick up and transport patients already cannulated. An understanding of the process of cannulation will aide in the care of these patients.
Locations for cannulation can include the operating room, Cardiac Cath lab, Interventional Radiology suite, or at the bedside. Depending on the facility, fluoroscopy may be utilized. Other options for cannulation assistance include TEE, TTE, and x-ray. Regardless of the cannulation technique used, a confirmatory x-ray should be performed prior to transport to ensure that cannulas are in good position. Also, check to make sure that mixing is not occurring (discussed later). For the placement of the dual lumen cannula, fluoroscopy is the preferred method of assuring bicaval position though echocardiogram can also be used. These cannulas are typically not placed at the bedside but transport providers may be asked to transport patients with this type of cannulation so familiarization is important.
The two cannula (right IJ, right femoral) system is the most common technique and can be accomplished at the bedside using echo and confirmed with x-ray. Below are the appropriate positions for the cannulas:
RIJ/R Fem: The right IJ return cannula sits at the level of the entrance of the right atrium. The right femoral cannula sits at the level of the IVC
Dual lumen right IJ: The “drainage” lumen sits in the IVC while the return lumen sits in the right atrium with the jet pointed towards the tricuspid valve
Fem/Fem cannulation: Ensure that there is no mixing and that the return cannula extends further up the IVC than the drainage cannula
Ultrasound guided access and cannulation of the vessels via seldinger technique is a popular approach though cutdown on the femoral vessels may still sometimes be used. Cannulas should be sutured into place and a dedicated individual should be responsible for cannulas and tubing during any movement.
Initial Considerations when Going on Circuit:
Initial Cannulation:
While the proceduralists are performing the cannulation, a dedicated individual should be responsible for medications and monitoring of the patients hemodynamics. These patients are often hypoxic and on maximum ventilator settings prior to cannulation, making lying flat for a cannulation difficult. Desaturation during initial cannulation can lead to further hemodynamic compromise in the form of arrhythmias and hypotension.
Prior to placement of cannulas, a heparin bolus should be administered. Though patients on VV ECMO typically do not require as aggressive early anticoagulation as their VA counterparts, the initial cannulation is considered to be pro-thrombotic with new external devices being introduced and there should be a bolus of anticoagulation. With a therapeutic ACT, a heparin gtt does not necessarily need started prior to transport. The typical goal PTT range on VV ECMO is 60-80.
Push dose vasopressors should also be immediately available as these patients can become hypotensive with hypoxia. In addition, if the patient is thought to be hypovolemic, crystalloid, albumin, and blood product should all be immediately available at the bedside for administration.
After cannulation placement, we recommend an x-ray for confirmation of cannula position prior to movement.
Circuit Change:
On arrival, some patients may already be cannulated. These patients will require a transition to the transport team circuit. Similar to above, these patients may have hypoxic, hypotensive episodes and all of the above precautions for initial cannulation should also be considered for a circuit change.
Transportation:
After the patient has been stabilized on the transport ECMO circuit, care should be taken with any movement. Just as there is a dedicated provider for the endotracheal tube, a dedicated provider should also monitor the cannulas. Check hemodynamics and ECMO flow parameters before and after each movement.
The ability to monitor blood gas values is particularly helpful, especially for longer transports, so that sweep and flow can be titrated to adjust pCO2 and pO2 levels. With an adequate blood gas, patients can be placed on “rest” ventilator settings (lowering PEEP and FiO2 in a slow, stepwise fashion). We do not recommend titrating down these values too quickly while transporting to prevent acidosis and hypoxia.
The receiving facility should be updated on any infusions as well as the ECMO parameters (flow, sweep, RPM) so that they may be prepared to receive the patient on your arrival.
Managing Complications:
Recirculation: Recirculation is when oxygenated blood from the circuit is removed through the drainage cannula prior to having the opportunity to enter the systemic circulation. This is affected by:
Cannula position: If the return jet is in too close in proximity to the drainage cannula. Increasing the distance between the cannulae can be of assistance if this is the issue with recirculation.
Increased intra-thoracic, cardiac pressure: impedes venous return to the heart which may direct oxygenated blood to the drainage cannula and away from the systemic circulation
Recirculation (%) = (SpreO2 – SvO2)/(SpostO2 – SvO2) x100
An increased SvO2 on the circuit with a persistent low arterial pO2 when checked on blood gas is a good indication that recirculation is occurring. If the patient’s pO2 is adequate and oxygen saturations are appropriate, the patient may still be safe for transport with some recirculation. Consultation with the accepting ECMO center may be helpful.
High negative pressures: Negative pressure is measured on the ECMO circuit. The pre-oxygenator pressure can be affected by:
Patient volume status
Type of cannula
Increasingly negative values can lead to suction events against the vessel and loss of flow. Volume can be considered but the type of cannula and position should also be considered. If possible, speed and flow can be decreased while the patient is treated.
Cannula dislodgement: Cannula dislodgement is a dreaded complication of transport on ECMO. A dedicated provider should monitor the cannulas throughout any movement. Prior to leaving, some type of imaging should be performed to assess for appropriate cannula placement. This is usually an x-ray. If cannulas are dislodged, direct pressure should be held and the patient’s ventilator should be maximized.
Equipment:
We carry a standard set of equipment for ECMO cannulation. Knowing the weights of all equipment transported is particularly important with any program that has an aviation component.
1.) Case for ECMO cannulation and circuit equipment- 9.8 kg 2.) Medication bag- 2 kg 3.) Additional ECMO cannulation equipment- 3 kg 4.)Circuit- 12 kg
AUTHORED BY LIZ POWELL, MD; PAIGE BARGER, ACNP; ADAM GOTTULA, MD
Dr. Powell is an Emergency Medicine attending and Critical Care fellow at the University of Cincinnati. Ms. Barger is an Anesthesia NP with the Cardiology Consult service. Dr. Gottula is a third-year Emergency Medicine resident at the University of Cincinnati
FACULTY EDITORS GRETCHEN LEMMINK, MD
Dr. Lemmink is an attending Anesthesiologist and critical care physician and fellowship director of Anesthesia Critical Care at the University of Cincinnati
References:
ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support Extracorporeal Life Support Organization, Version 1.4 August 2017 Ann Arbor, MI, USA
Pappalardo, Federico, et al. “Predicting Mortality Risk in Patients Undergoing Venovenous ECMO for ARDS Due to Influenza A (H1N1) Pneumonia: the ECMOnet Score.” Intensive Care Medicine, vol. 39, no. 2, 2012, pp. 275–281., doi:10.1007/s00134-012-2747-1.
Peek, Giles J, et al. “Efficacy and Economic Assessment of Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR): a Multicentre Randomised Controlled Trial.” The Lancet, vol. 374, no. 9698, 2009, pp. 1351–1363., doi:10.1016/s0140-6736(09)61069-2
Combes, Alain, et al. “Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome.” New England Journal of Medicine, vol. 378, no. 21, 2018, pp. 1965–1975., doi:10.1056/nejmoa1800385