The CLOVERS Trial
/National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network, Shapiro NI, Douglas IS, et al. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 2023;388(6):499-510. doi:10.1056/NEJMoa2212663
BACKGROUND
Sepsis, including severe sepsis and septic shock, is a frequently encountered condition in the emergency department and carries a high mortality rate. One of the primary pathophysiologic mechanisms involves complex cascade of host dysregulation in response to an infectious stimulus (Evans, Rhodes et al. 2021, Jarczak, Kluge et al. 2021). Recent meta-analyses and systematic reviews evaluating mortality in patients with septic shock reported mortality as high as 35% and 38% at 30 and 90 days, respectively (Vincent, Jones et al. 2019, Bauer, Gerlach et al. 2020). Despite the complexity and heterogeneity of patients with sepsis, there have been few interventions which have been demonstrated to decrease mortality: early antimicrobial and fluid administration (Levy, Evans et al. 2018, Kuttab, Lykins et al. 2019, Evans, Rhodes et al. 2021, Im, Kang et al. 2022), ideally with antibiotics administered within one hour of sepsis recognition by the treating provider (Evans, Rhodes et al. 2021). Each subsequent one-hour delay in antimicrobial administration increases mortality by 35% in patients with septic shock (Im, Kang et al. 2022).
The SSC guidelines recommend administering at least 30mL/kg of crystalloid within a three hour period for patients with sepsis-associated hypoperfusion and septic shock (Evans, Rhodes et al. 2021), although this is based on low-quality evidence rather than high-quality randomized trials and has remained a controversial aspect of treatment of patients with sepsis given the potential harms of large-volume fluid administration. In fact, specifically within the SSC guidelines the evidence is described verbatim as “weak recommendation, low-quality evidence” (Evans, Rhodes et al. 2021).
Several studies have begun exploring the potential link between large-volume fluid administration and mortality and end-organ harm in patients with sepsis and septic shock. Respiratory failure and more severe acute kidney injury were demonstrated to be more common in patients who received higher volumes of intravenous fluid (Andrews, Muchemwa et al. 2014, Hjortrup, Haase et al. 2016, Andrews, Semler et al. 2017, Silversides, Major et al. 2017). A systematic review and meta-analysis of several cohort studies demonstrated 70% increased mortality (pooled RR: 1.70; CI: 1.20, 2.41; P = .003) with high-volume fluid administration in patients with severe sepsis and septic shock, and significantly lower mortality with low-volume fluid administration in the first 24h of treatment (P=0.02) (Tigabu, Davari et al. 2018). This was corroborated by another systematic review which demonstrated decreased mortality in restrictive compared to liberal fluid administration strategies (24.7% vs 33.2%; OR, 0.42; 95% CI 0.32-0.55; P < 0.0001) (Malbrain, Marik et al. 2014).
The ANDROMEDA-SHOCK trial published in 2019 demonstrated that only one-quarter (25%) of patients were fluid-responsive at baseline, as determined most frequently by velocity time integral or passive leg raise methods (Hernández, Ospina-Tascón et al. 2019). In 2022, the CLASSIC trial was published which provided one investigational angle to this important question. The CLASSIC trial is a randomized controlled trial conducted in the ICU setting which compared restrictive versus standard fluid strategies with a primary outcome of death by 90 days in patients with septic shock. This trial involved employing a restrictive versus standard strategy with the restrictive fluid strategy permitting fluid boluses only when the patient met one of several criteria, whereas the standard fluid strategy had no limit to fluid quantity administered. Mortality at 90 days was equivocal between groups (42.3% vs 42.1%), as were days alive outside of the hospital and days without life support (Meyhoff, Hjortrup et al. 2022).
The CLASSIC trial nicely set up for the introduction and publishing of the primary study of focus of this post:
The CLOVERS 🍀 Trial
(National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network, Shapiro NI, Douglas IS, et al. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 2023;388(6):499-510. doi:10.1056/NEJMoa2212663)
METHODS
Setting
The CLOVERS trial is a multi-center, unblinded, superiority randomized controlled trial which functioned as part of the PETAL (Prevention and Early Treatment of Acute Lung Injury Network) network. Sixty centers participated during the years 2018-2022.
Inclusion criteria
Adults (18 years and older) with “suspected or confirmed infection” with sepsis-induced hypotension, which was defined as SBP less than 100 after 1L or greater of crystalloid administration, were included.
Exclusion criteria
Patients were excluded if:
It had been greater than four hours since meeting criteria for sepsis-induced hypotension (SBP <100 after 1L≤ crystalloid administered).
Greater than 24h since presentation to the hospital
Having received greater than 3L intravenous fluids – including prehospital volume administration
Evidence of volume overload (importantly, did not exclude patients carrying a diagnosis of heart failure or end-stage renal disease by diagnosis alone)
Evidence of severe volume depletion for other causes (ex. elderly patient with HHS found down with severe hypovolemic shock, hypernatremia, etc.)
Randomization
This was accomplished via a Web-based centralized system with site-based stratification.
Intervention
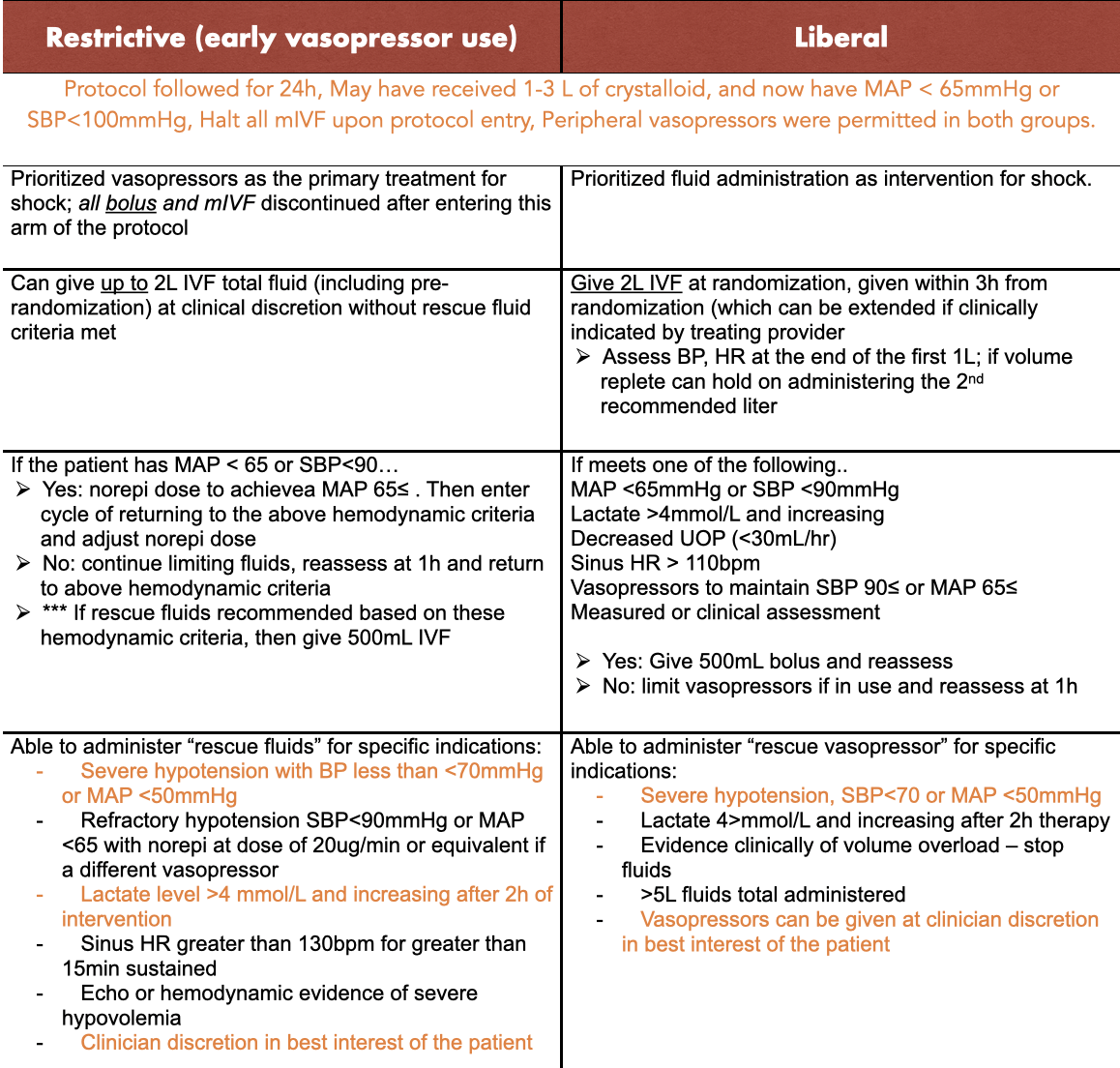
Protocol
Adherence was amended in October of 2019 to limit administered fluid bolus to 1L if vital signs had stabilized (noted in chart above). The study was stopped at the second interim analysis due to futility – rather than harm from the DSMB. However, protocol adherence was monitored for the first 300 patients and during the remainder of the trial.
Study power
They assumed at baseline mortality of 15% and the study was powered to detect an absolute difference of 4.5% in the restrictive fluid group. 2,320 patients were needed to have 90% power at a significance level of 0.05.
RESULTS
A total of 1,563 patients were enrolled with 782 assigned to the restrictive and 781 assigned to the liberal fluid groups. There were similar baseline characteristics and the groups were well-matched. Before randomization, both groups received similar fluid volumes (median 2050mL for both) and similar percentages of vasopressor use (21% in restrictive, 18% in liberal).
Fluid volumes between groups at the six-hour mark differed – with a median of 500mL and 2300mL in the restrictive and liberal fluid groups respectively. At the 24h mark, the median volumes were 1267mL and 3400mL (restrictive, liberal) and mean difference of -2134mL. 59% of patients in the restrictive and 37% in liberal fluid groups received vasopressors. Lactated ringer’s solution was the most frequently chosen fluid type.
Adherence
Protocol adherence was remarkably high – 97% vs 96% in the restrictive and liberal groups and sustained throughout the duration of the trial.
Post-hoc analysis
There were some post-hoc analyses that were conducted including more patients in the restrictive group were admitted to the intensive care unit versus the liberal group (67.3% versus 59.2%). No effects stratified by site were detected.
Primary outcome
There was no significant difference in the primary outcome, death before discharge home by 90 days between groups (14.0% versus 14.9% in restrictive versus liberal; estimated difference -0.9 percentage points; 95% CI -4.4 to 2.6; P=0.61). Subgroup analyses which were prespecified such as chronic heart failure, ESRD, pneumonia as cause, and receipt of vasopressors or SBP<90mmHg at randomization were conducted and reported in detail in the primary literature, but demonstrated interestingly a mortality difference of -20.2% for patients with ESRD in the restrictive group (95% CI; -41.9 to 1.5).
Safety outcomes
Serious adverse event occurrences were similar in both groups. For both volume overload and pulmonary edema, each occurred only 3 times in the liberal group versus 0 in the restrictive group. There were only three possible events of vasopressor extravasation, among 500 patients, who did receive vasopressors, all of which resolved without intervention and left no residual effects on the patient.
DISCUSSION
This was a well-conducted randomize controlled trial evaluating an important clinical question, and importantly, was conducted in an emergency department setting and has a significant strength and addition to the literature in that this is a prospective trial. It would be difficult to perform a blinded trial of similar nature – although this does provide for some limitation on potential biases. Unfortunately, it was ultimately underpowered and enrollment was ceased early secondary to futility as determined by the DSMB. Additionally, some other studies have identified a higher mortality than prespecified in the methods section (15%) – closer to ~35% - and raises the question of similarity to some patient populations, which may be much more critically ill at baseline. The protocol was well-designed, however also had to weigh the ml/kg versus absolute fluid volume for standardization; would 30mL/kg have been a better initial comparator group rather than absolute untailored fluid amounts, based on the Surviving Sepsis Campaign guidelines?
A 90-day timeline outcome was used similarly as an outcome to the CLASSIC trial; this is a patient-centered outcome, and corroborates the emergency department counterpiece to the ICU-based CLASSIC trial suggesting no difference in 90-day discharge home and 90-day mortality between the two trials. However – is this the best endpoint for such a trial? 90 days accounts for significant post-treatment (given 24h protocol) variability which is influenced by a grand multitude of factors.
Both restrictive and more liberal protocols for the treatment of septic shock appear to be viable initial resuscitation strategies, and importantly, safety was maintained in both – including for the peripheral administration of vasopressors which is a key point for emergency providers who may have limitations on time and bandwidth in caring for many patients simultaneously in the emergency department and could limit performing unnecessary central lines and invasive procedures on patients. It would be interesting to stratify the doses of vasopressor administration to the few potential adverse events (all of which resolved), or the location of the peripheral line, given the questions raised regarding safety and patency of ultrasound-guided IVs versus non-ultrasound guided-IVs.
Specific patient populations were excluded, of note, such as the septic, already-volume-overloaded or severely hypovolemic patient – these will require careful, separate consideration in the tailored, specialized treatment of septic shock. There was also an advantage of using MAP as an endpoint, which is a significant advantage as this is a standard resuscitation and hemodynamic end point in routine clinical care of patients in shock. Although protocol adherence was high, one challenge that is pervasive between studies of similar hypothesis regarding fluid management, is heterogeneity of the treatment effects. There was significant crossover in baseline vasopressor administration, for example – and although this is appropriate for patient safety and for clinical care flexibility, it does somewhat cloud the ultimate interpretation and treatment effects.
Overall, both restrictive and liberal fluid strategies in the initial resuscitation of patients with septic shock, when applied to the appropriate patient populations, are viable strategies for care based on the provided endpoints and subgroup and post-hoc analyses. This study raised some interesting potential questions and hypotheses for future studies based on the subgroup analyses, and demonstrated excellent safety for the administration of peripheral vasopressors.
REFERENCES
Andrews, B., L. Muchemwa, P. Kelly, S. Lakhi, D. C. Heimburger and G. R. Bernard (2014). "Simplified severe sepsis protocol: a randomized controlled trial of modified early goal-directed therapy in Zambia." Crit Care Med 42(11): 2315-2324.
Andrews, B., M. W. Semler, L. Muchemwa, P. Kelly, S. Lakhi, D. C. Heimburger, C. Mabula, M. Bwalya and G. R. Bernard (2017). "Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial." Jama 318(13): 1233-1240.
Bauer, M., H. Gerlach, T. Vogelmann, F. Preissing, J. Stiefel and D. Adam (2020). "Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis."Crit Care 24(1): 239.
Evans, L., A. Rhodes, W. Alhazzani, M. Antonelli, C. M. Coopersmith, C. French, F. R. Machado, L. McIntyre, M. Ostermann, H. C. Prescott, C. Schorr, S. Simpson, W. J. Wiersinga, F. Alshamsi, D. C. Angus, Y. Arabi, L. Azevedo, R. Beale, G. Beilman, E. Belley-Cote, L. Burry, M. Cecconi, J. Centofanti, A. Coz Yataco, J. De Waele, R. P. Dellinger, K. Doi, B. Du, E. Estenssoro, R. Ferrer, C. Gomersall, C. Hodgson, M. H. Moller, T. Iwashyna, S. Jacob, R. Kleinpell, M. Klompas, Y. Koh, A. Kumar, A. Kwizera, S. Lobo, H. Masur, S. McGloughlin, S. Mehta, Y. Mehta, M. Mer, M. Nunnally, S. Oczkowski, T. Osborn, E. Papathanassoglou, A. Perner, M. Puskarich, J. Roberts, W. Schweickert, M. Seckel, J. Sevransky, C. L. Sprung, T. Welte, J. Zimmerman and M. Levy (2021). "Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021." Intensive Care Med 47(11): 1181-1247.
Hernández, G., G. A. Ospina-Tascón, L. P. Damiani, E. Estenssoro, A. Dubin, J. Hurtado, G. Friedman, R. Castro, L. Alegría, J. L. Teboul, M. Cecconi, G. Ferri, M. Jibaja, R. Pairumani, P. Fernández, D. Barahona, V. Granda-Luna, A. B. Cavalcanti, J. Bakker, G. Hernández, G. Ospina-Tascón, L. Petri Damiani, E. Estenssoro, A. Dubin, J. Hurtado, G. Friedman, R. Castro, L. Alegría, J. L. Teboul, M. Cecconi, M. Cecconi, G. Ferri, M. Jibaja, R. Pairumani, P. Fernández, D. Barahona, A. B. Cavalcanti, J. Bakker, G. Hernández, L. Alegría, G. Ferri, N. Rodriguez, P. Holger, N. Soto, M. Pozo, J. Bakker, D. Cook, J. L. Vincent, A. Rhodes, B. P. Kavanagh, P. Dellinger, W. Rietdijk, D. Carpio, N. Pavéz, E. Henriquez, S. Bravo, E. D. Valenzuela, M. Vera, J. Dreyse, V. Oviedo, M. A. Cid, M. Larroulet, E. Petruska, C. Sarabia, D. Gallardo, J. E. Sanchez, H. González, J. M. Arancibia, A. Muñoz, G. Ramirez, F. Aravena, A. Aquevedo, F. Zambrano, M. Bozinovic, F. Valle, M. Ramirez, V. Rossel, P. Muñoz, C. Ceballos, C. Esveile, C. Carmona, E. Candia, D. Mendoza, A. Sanchez, D. Ponce, D. Ponce, J. Lastra, B. Nahuelpán, F. Fasce, C. Luengo, N. Medel, C. Cortés, L. Campassi, P. Rubatto, N. Horna, M. Furche, J. C. Pendino, L. Bettini, C. Lovesio, M. C. González, J. Rodruguez, H. Canales, F. Caminos, C. Galletti, E. Minoldo, M. J. Aramburu, D. Olmos, N. Nin, J. Tenzi, C. Quiroga, P. Lacuesta, A. Gaudín, R. Pais, A. Silvestre, G. Olivera, G. Rieppi, D. Berrutti, M. Ochoa, P. Cobos, F. Vintimilla, V. Ramirez, M. Tobar, F. García, F. Picoita, N. Remache, V. Granda, F. Paredes, E. Barzallo, P. Garcés, F. Guerrero, S. Salazar, G. Torres, C. Tana, J. Calahorrano, F. Solis, P. Torres, L. Herrera, A. Ornes, V. Peréz, G. Delgado, A. López, E. Espinosa, J. Moreira, B. Salcedo, I. Villacres, J. Suing, M. Lopez, L. Gomez, G. Toctaquiza, M. Cadena Zapata, M. A. Orazabal, R. Pardo Espejo, J. Jimenez, A. Calderón, G. Paredes, J. L. Barberán, T. Moya, H. Atehortua, R. Sabogal, G. Ortiz, A. Lara, F. Sanchez, A. Hernán Portilla, H. Dávila, J. A. Mora, L. E. Calderón, I. Alvarez, E. Escobar, A. Bejarano, L. A. Bustamante and J. L. Aldana (2019). "Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial." Jama 321(7): 654-664.
Hjortrup, P. B., N. Haase, H. Bundgaard, S. L. Thomsen, R. Winding, V. Pettilä, A. Aaen, D. Lodahl, R. E. Berthelsen, H. Christensen, M. B. Madsen, P. Winkel, J. Wetterslev and A. Perner (2016). "Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial." Intensive Care Med 42(11): 1695-1705.
Im, Y., D. Kang, R. E. Ko, Y. J. Lee, S. Y. Lim, S. Park, S. J. Na, C. R. Chung, M. H. Park, D. K. Oh, C. M. Lim, G. Y. Suh and i. Korean Sepsis Alliance (2022). "Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: a prospective nationwide multicenter cohort study." Crit Care 26(1): 19.
Jarczak, D., S. Kluge and A. Nierhaus (2021). "Sepsis-Pathophysiology and Therapeutic Concepts." Front Med (Lausanne) 8: 628302.
Kuttab, H. I., J. D. Lykins, M. D. Hughes, K. Wroblewski, E. P. Keast, O. Kukoyi, J. A. Kopec, S. Hall and M. A. Ward (2019). "Evaluation and Predictors of Fluid Resuscitation in Patients With Severe Sepsis and Septic Shock." Crit Care Med 47(11): 1582-1590.
Levy, M. M., L. E. Evans and A. Rhodes (2018). "The Surviving Sepsis Campaign Bundle: 2018 update." Intensive Care Med 44(6): 925-928.
Malbrain, M. L., P. E. Marik, I. Witters, C. Cordemans, A. W. Kirkpatrick, D. J. Roberts and N. Van Regenmortel (2014). "Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice." Anaesthesiol Intensive Ther 46(5): 361-380.
Meyhoff, T. S., P. B. Hjortrup, J. Wetterslev, P. Sivapalan, J. H. Laake, M. Cronhjort, S. M. Jakob, M. Cecconi, M. Nalos, M. Ostermann, M. Malbrain, V. Pettila, M. H. Moller, M. N. Kjaer, T. Lange, C. Overgaard-Steensen, B. A. Brand, M. Winther-Olesen, J. O. White, L. Quist, B. Westergaard, A. B. Jonsson, C. J. S. Hjortso, N. Meier, T. S. Jensen, J. Engstrom, L. Nebrich, N. C. Andersen-Ranberg, J. V. Jensen, N. A. Joseph, L. M. Poulsen, L. S. Herlov, C. G. Solling, S. K. Pedersen, K. K. Knudsen, T. S. Straarup, M. L. Vang, H. Bundgaard, B. S. Rasmussen, S. R. Aagaard, T. Hildebrandt, L. Russell, M. H. Bestle, M. Schonemann-Lund, A. C. Brochner, C. F. Elvander, S. K. L. Hoffmann, M. L. Rasmussen, Y. K. Martin, F. F. Friberg, H. Seter, T. N. Aslam, S. Adnoy, P. Seidel, K. Strand, B. Johnstad, E. Joelsson-Alm, J. Christensen, C. Ahlstedt, C. A. Pfortmueller, M. Siegemund, M. Greco, J. Radej, M. Kriz, D. W. Gould, K. M. Rowan, P. R. Mouncey, A. Perner and C. T. Group (2022). "Restriction of Intravenous Fluid in ICU Patients with Septic Shock." N Engl J Med 386(26): 2459-2470.
Silversides, J. A., E. Major, A. J. Ferguson, E. E. Mann, D. F. McAuley, J. C. Marshall, B. Blackwood and E. Fan (2017). "Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis." Intensive Care Med 43(2): 155-170.
Tigabu, B. M., M. Davari, A. Kebriaeezadeh and M. Mojtahedzadeh (2018). "Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review." J Crit Care 48: 153-159.
Vincent, J. L., G. Jones, S. David, E. Olariu and K. K. Cadwell (2019). "Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis." Crit Care 23(1): 196.
Authorship
Written by Lauren Gillespie, MD, PGY-3, University of Cincinnati Department of Emergency Medicine
Peer Review, Editing, Audio Editing, Posting by Jeffery Hill, MD MEd
Cite As
Gillespie, L., Hill, J. (May 5, 2023) The CLOVERS Trial. TamingtheSRU. https://www.tamingthesru.com/blog/journal-club/the-clovers-trial