Ultrasound of the Month: Aortic Dissection
/The patient is a middle year old male with a history of hypertension who presents with a chief complaint of chest pain. He reports onset of pain in the center of his chest, beginning one hour prior to presentation, described as a “burning” sensation associated with mild epigastric pain. The pain began as a sharp pain, but now resembles a burning sensation that he says is similar to heartburn. It radiates to his back and is 4/10 in severity. He also notes tingling in his left upper extremity that was present at initial symptom onset but has resolved. On physical examination, he is well appearing and in no acute distress. Heart and lung auscultation are unremarkable. He has 2+ pulses in all four extremities and has a non-focal neurologic examination. His labs are remarkable for leukocytosis to 15,000 and an elevated d-dimer to 10.57 ug/dL. Otherwise, his labs indicate a normal basic metabolic panel, high sensitivity troponin of 12 (ref 0-12), normal INR and VBG. A chest X-ray is performed and is read as normal. Electrocardiogram demonstrates normal sinus rhythm with T-wave inversions in avL, V5 and V6, with evidence of left ventricular hypertrophy.
Point of care ultrasound (POCUS) was performed which demonstrated the following:
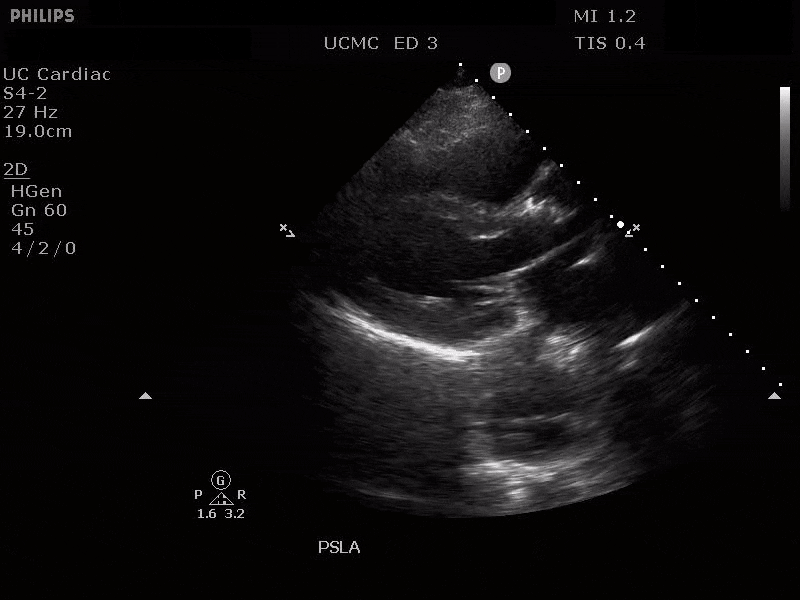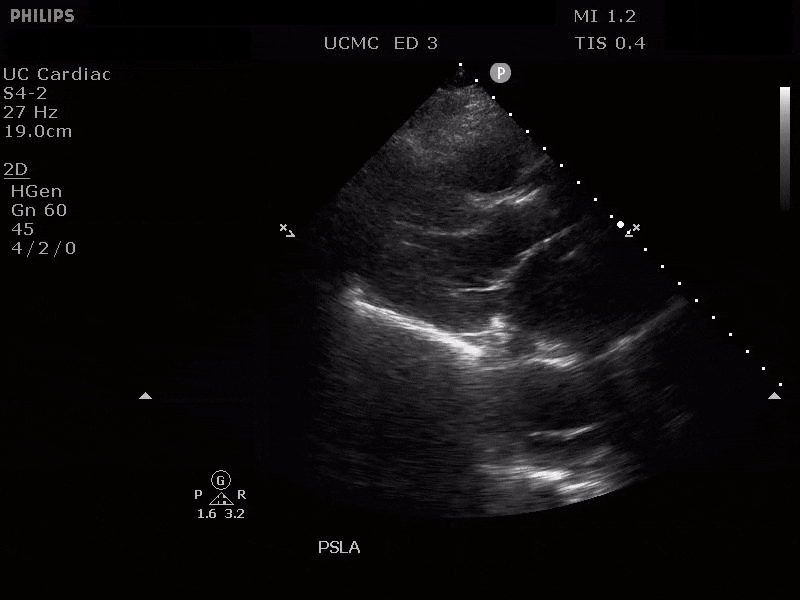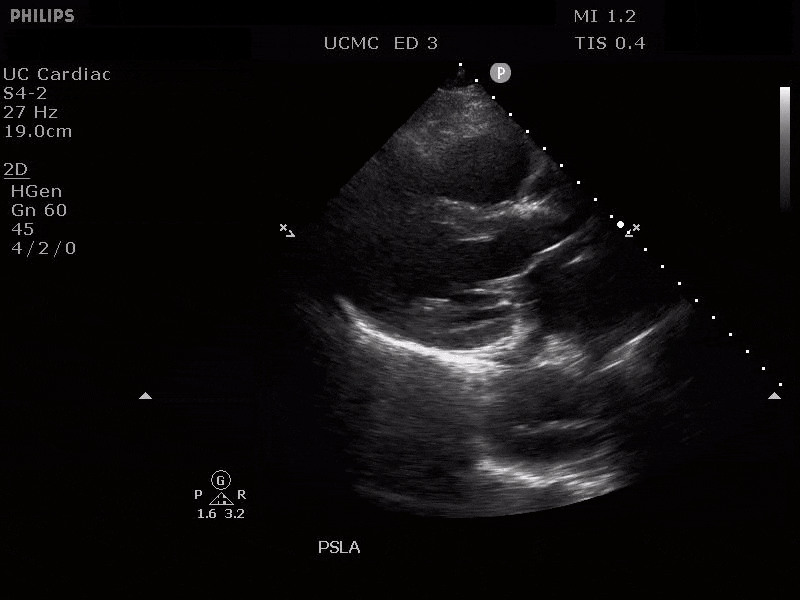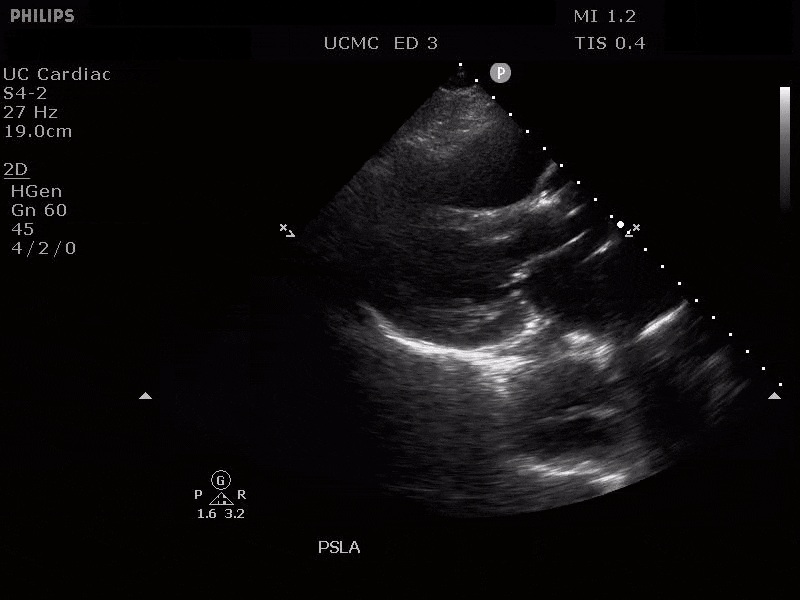
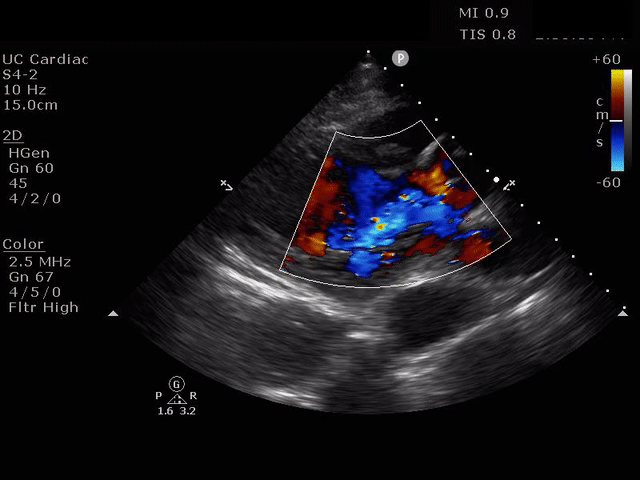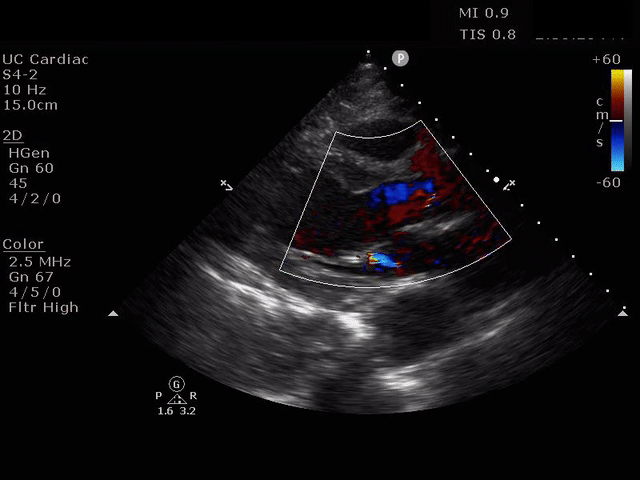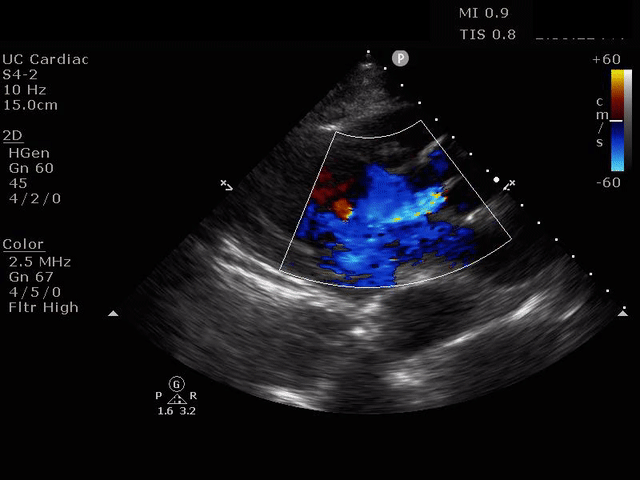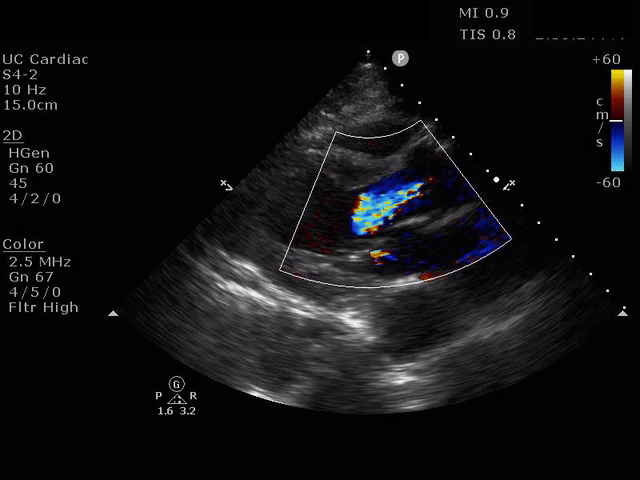
Parasternal long axis view with dissection flap visible in the proximal ascending aorta
Parasternal short axis view with dissection flap visible in the descending thoracic aorta
The parasternal short axis view demonstrates evidence of a mobile hyperechoic structure within an anechoic tubular structure deep to the heart, which represents a dissection flap within the descending aorta. The parasternal long view demonstrates a dilated proximal aorta with a dissection flap originating at the aortic root. Aortic dissection is a challenging diagnosis to make, and the remainder of this article will discuss the bedside evaluation for aortic dissection with point of care ultrasound.
Aortic dissection (AD) is a life-threatening condition caused by the separation of the intimal layer of the aorta from the medial layer, allowing blood to penetrate the media and create a false plane that can occlude the aortic lumen and its branches. The area of intimal rupture most often occurs within a few centimeters of the aortic valve on the right lateral wall of the ascending aorta or close to the ligamentum arteriosum in the descending aorta (1). Sixty-five percent of cases occur within 3 cm of the coronary ostia, 10% occur within the arch, and 10% in the descending thoracic aorta. Type A dissections involve portions of the ascending aorta while Type B dissections are limited to the descending aorta. Mortality is high for Type A dissections, accumulating 1-2% mortality per hour during the first 24-48 hours (2). AD is a rare occurrence, with an annual incidence of 10 to 30 cases per million patients per year (3).
AD is often a difficult diagnosis to make. In a 27-year survey of AD cases, the correct diagnosis was only made during 15% of all workups prior to autopsy diagnosis (4). Symptoms can vary widely, ranging from severe, tearing or ripping pain radiating from the chest to the back and abdomen, to 10% of patients experiencing no pain at all (5,6). The physical exam is also often unreliable, as the classic teaching of asymmetric upper extremity pulses is only present in 15% of cases (6). Aortic regurgitation is also only present in about 30% of cases by auscultation. Furthermore, chest X-ray has been found to be incompletely sensitive for AD, with widened mediastinum present in approximately 60% of cases. Electrocardiography is also normal in 30% of cases (7).
The European Society of Cardiology recommends multidetector computed tomography (CT) angiography as the first line of investigation for patients with suspected acute dissection, as it carries a sensitivity of nearly 100% and specificity of 98% (8). Magnetic resonance imaging (MRI) also possesses a high sensitivity and specificity of 98% and 98%. Transesophageal echocardiography (TEE) is an alternate diagnostic modality, with sensitivity of 98% and specificity 95%. In the absence of these imaging modalities, conventional spiral CT with transthoracic echocardiogram (TTE) is a useful means to assess for AD (8). However, TTE alone may be insufficient to diagnose AD, as it has been reported to have a sensitivity of 67-90% and a specificity of 71-100% for this diagnosis (9).
Parasternal long axis view with dilated descending thoracic aorta and visible dissection flap
Limitations of Ultrasound:
TTE can visualize dissection flaps within the proximal 4 to 8 mm of the ascending aorta as well as a short segment of the descending aorta, but has poor sensitivity for all dissections in the descending aorta (31-55%) (1,10). To diagnose an aortic dissection, a hyperechoic dissection flap must be visualized within the walls of the aorta, dividing the cavity into true and false lumens (9). Color doppler can be applied to these spaces to differentiate the true from the false lumen. In most cases, the false lumen has poor flow whereas the true lumen should have brisk antegrade flow during systole with movement of the dissection flap toward the false lumen (1). If no color flow is identified within a cavity, it may suggest thrombosis of the false lumen. However, the operator must be careful not to image at a 90 degree angle of insonation, which may also cause a falsely negative doppler signal.
POCUS Assessment for Aortic Dissection:
Despite its variable test characteristics, TTE is useful for rapid bedside assessment for complications of aortic dissection: identifying aortic valve dilation, aortic regurgitation, regional wall motion abnormalities, pericardial effusion and hemothorax. The presence of these findings is concerning for a Type A dissection, but their absence does not rule out the diagnosis (1).
The presence of a dilated aortic root and aortic regurgitation is important because it has direct implications on surgical planning. Aortic root size varies widely among gender and also individual BMI. The sinus of valsalva is usually the widest portion of the aortic root and should not measure larger than 4.5 cm in diameter (11,12,13). The aortic root is best measured in the parasternal long axis, and most accurate measurements are taken using the zoom function over the area of interest. The aortic annulus is measured at the base of the aortic valves at mid systole, and should be measured inner-edge to inner-edge with a normal range between 2-3.1 cm in diameter (13). The structures above the aortic valve (sinus of valsalva, sinotubular junction, ascending aorta) are measured differently; they are measured at end diastole, and are measured leading edge to leading edge, which spans one inner wall to the opposing outer wall and includes the wall thickness. Additionally, use of color doppler through the aortic valve can help identify the presence of aortic regurgitation, which can be visualized in 40-76% of cases (13). A regurgitant jet originating from the aortic valve into the left ventricle during diastole is characteristic of aortic regurgitation, which can be a consequence of aortic root dilation and AD.
Parasternal Long Axis view with Aortic Regurgitation
The presence of regional wall motion abnormalities (particularly inferior hypokinesis), may suggest occlusion of the coronary arteries from a dissection flap. 10-15% of cases of AD will occlude the coronary arteries, with the right coronary artery being most frequently affected (13). Regional wall motion abnormalities are best visualized in the parasternal short axis (see this TTS post). Lastly, the presence of pericardial effusion or hemothorax suggests impending aortic rupture. This will appear as an anechoic or hypoechoic stripe surrounding the heart, best visualized in the parasternal or subcostal views. Hemothoraces appear as anechoic spaces surrounding the inferior lung, also evidenced by continuation of the spine past the diaphragm (“spine sign”). Discovery of these findings in the presence of a dissection flap should lead to expeditious surgical consultation and advanced imaging (CTA, MRI or intraoperative TEE) when possible.
Back to the case:
This patient was found to have an aortic dissection flap visible on bedside echocardiogram. A CT angiogram confirmed the diagnosis and revealed a Type A dissection that extended distally into the iliac arteries. Cardiothoracic surgery was urgently consulted and the patient was started on antihypertensives and beta blockers. The patient underwent replacement of his aortic arch with re-implantation of his cranial vessels. The patient did well through the surgery and was discharged a few weeks later.
AUTHORED BY Arthur Broadstock, MD
Dr. Broadstock (@BroadstockMD) is a PGY-3 in Emergency Medicine at the University of Cincinnati and Resident Editor of Ultrasound of the Month.
PEER REVIEW BY JESSICA BAEZ, MD AND LORI STOLZ, MD RDMS
Dr. Baez (@blonde_doctr) is an Assistant Professor of Emergency Medicine at the University of Cincinnati and Assistant Residency Director.
Dr. Stolz (@sonostolz) is an Associate Professor of Emergency Medicine at the University of Cincinnati and Director of the Ultrasound Fellowship.
References:
Meredith, E. L., & Masani, N. D. (2009). Echocardiography in the emergency assessment of acute aortic syndromes. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology, 10(1), i31–i39. https://doi.org/10.1093/ejechocard/jen251
Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17(4):615-vii. doi:10.1016/s0733-8651(05)70105-3.
Baliga RR, Nienaber CA, Bossone E, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging. 2014;7(4):406-424. doi:10.1016/j.jcmg.2013.10.015.
Mészáros, I., Mórocz, J., Szlávi, J., Schmidt, J., Tornóci, L., Nagy, L., & Szép, L. (2000). Epidemiology and clinicopathology of aortic dissection. Chest, 117(5), 1271–1278. https://doi.org/10.1378/chest.117.5.1271
Crawford, E. S. (1990). The diagnosis and management of aortic dissection. JAMA, 264(19), 2537-2541.
Karthikesalingam, A., Holt, P. J. E., Hinchliffe, R. J., Thompson, M. M., & Loftus, I. M. (2010). The diagnosis and management of aortic dissection. Vascular and endovascular surgery, 44(3), 165-169.
Golledge J, Eagle KA. Acute aortic dissection. Lancet. 2008; 372(9632):55-66
Thrumurthy, S. G., Karthikesalingam, A., Patterson, B. O., Holt, P. J., & Thompson, M. M. (2012). The diagnosis and management of aortic dissection. BMJ, 344.
Fengju Liu, Lianjun Huang. Usefulness of ultrasound in the management of aortic dissection. Rev. Cardiovasc. Med. 2018, 19(3), 103–109. https://doi.org/10.31083/j.rcm.2018.03.3182
Baliga RR, Nienaber CA, Bossone E, et al. The role of imaging in aortic dissection and related syndromes. JACC Cardiovasc Imaging. 2014;7(4):406-424. doi:10.1016/j.jcmg.2013.10.015
Paruchuri, V., Salhab, K. F., Kuzmik, G., Gubernikoff, G., Fang, H., Rizzo, J. A., Ziganshin, B. A., & Elefteriades, J. A. (2015). Aortic Size Distribution in the General Population: Explaining the Size Paradox in Aortic Dissection. Cardiology, 131(4), 265–272. https://doi.org/10.1159/000381281
Pape, L. A., Tsai, T. T., Isselbacher, E. M., Oh, J. K., O'gara, P. T., Evangelista, A., Fattori, R., Meinhardt, G., Trimarchi, S., Bossone, E., Suzuki, T., Cooper, J. V., Froehlich, J. B., Nienaber, C. A., Eagle, K. A., & International Registry of Acute Aortic Dissection (IRAD) Investigators (2007). Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation, 116(10), 1120–1127. https://doi.org/10.1161/CIRCULATIONAHA.107.702720.
Evangelista, A., Flachskampf, F. A., Erbel, R., Antonini-Canterin, F., Vlachopoulos, C., Rocchi, G., Sicari, R., Nihoyannopoulos, P., Zamorano, J., European Association of Echocardiography, Document Reviewers:, Pepi, M., Breithardt, O. A., & Plonska-Gosciniak, E. (2010). Echocardiography in aortic diseases: EAE recommendations for clinical practice. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology, 11(8), 645–658. https://doi.org/10.1093/ejechocard/jeq056