Diagnostics and Therapeutics: Paracentesis in the Emergency Department
/Patients with cirrhosis and ascites may represent a small population of this country, but are often frequent utilizers of the emergency room due to the many morbid conditions associated with the disease process. Ascites (and need for removal) is one of the most frequent complications of cirrhosis, and therefore emergency physicians need to be well-versed in how to manage presentations to the emergency department. This post will review the procedure for removal of ascites – called a paracentesis – and discuss in depth indications, anatomy, equipment needed, performance of the procedure, and interpretation of results of fluid collected.
Overview: what is ascites and when do i need to remove it?
Ascites refers to the pathologic buildup of fluid in the peritoneal cavity. It is most often caused by portal hypertension from cirrhosis (1). Patients with cirrhotic ascites have a 3-year mortality rate of approximately 50%, and refractory ascites carries a 1-year survival rate of less than 50% (1). Ascites often needs to be removed for diagnostic and/or therapeutic purposes. Complications of ascites include spontaneous bacterial peritonitis, cellulitis, respiratory insufficiency via pleural effusions, reduced functional residual volume and atelectasis, abdominal wall hernias, hepato-renal syndrome, and chronic abdominal discomfort (2).
From a diagnostic standpoint, the most important diagnosis to exclude is spontaneous bacterial peritonitis (SBP), or acute infection of the ascitic fluid without an alternative surgically treatable source. SBP has been attributed to changes in the gut microbiome, intestinal permeability, and immune dysregulation in patients with cirrhotic ascites (2). Patients with SBP can present with abdominal pain, increasing abdominal distension, nausea, vomiting, fever, or altered mental status. However, up to 13% of SBP infections were subtle or even asymptomatic in one study (2). In-hospital mortality of SBP has been estimated to range from 20-50% and rises by 3% for every hour in delay in performing the paracentesis (2).
Alternatively, from a therapeutic standpoint, patients often present to the emergency department for removal of fluid simply due to abdominal discomfort and distention which when untreated can even lead to respiratory insufficiency from the abdominal cavity compressing the thorax and limiting diaphragmatic excursion. There are varying opinions within the emergency physician community on the amount of fluid that is safe to remove in the emergency department setting although the literature would suggest that removing up to 5 L of fluid in the emergency department is relatively safe (3,4).
LARGE-VOLUME PARACENTESIS
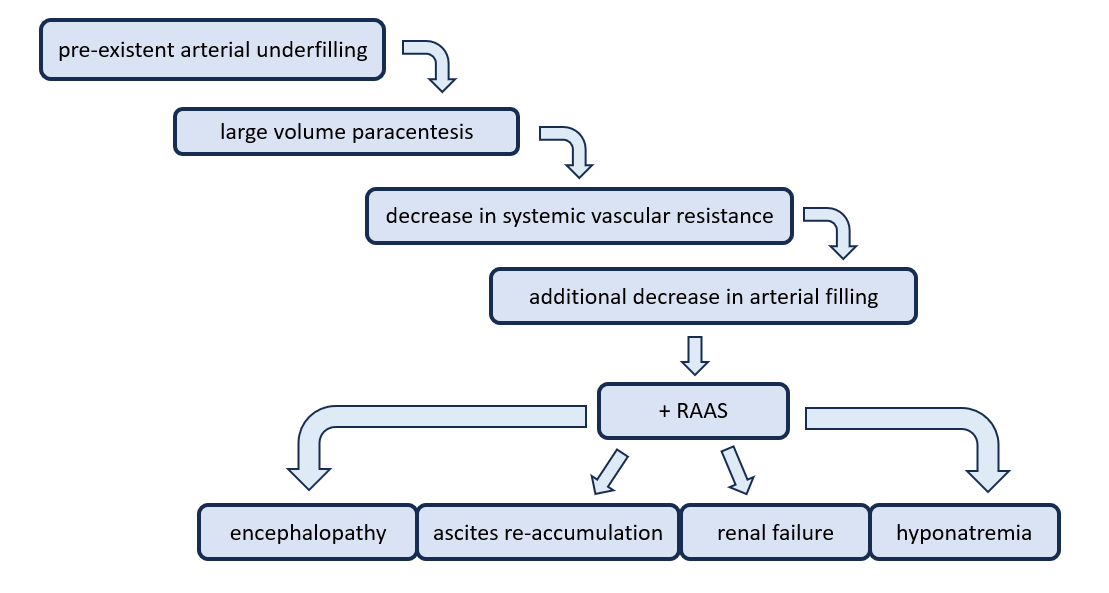
When more than 5 L of ascitic fluid is removed, this is referred to as a large volume paracentesis (LVP). This procedure is often reserved for patients with refractory ascites and can temporarily improve pulmonary status, decrease intra-abdominal hypertension, and eliminate abdominal discomfort (1). The most feared complication is paracentesis-induced circulatory dysfunction (PICD) which is when the body’s natural physiologic response to drastic fluid shifts is to rapidly re-distribute fluids. This can lead to rapid re-accumulation of ascitic fluid and intra-vascular depletion and subsequent hypotension. It is formally diagnosed when there is >50% increase in the baseline plasma renin activity to a value ≥4 ng/mL/h on the fifth to sixth day after paracentesis, but can also be diagnosed clinically by increasing abdominal distension and hypotension after large-volume paracentesis (5,6,7).
The current recommendation from the American Association for the Study of Liver Disease (AASLD) is to consider the administration of albumin (6-8 g/L of fluid removed) for patients undergoing removal of greater than 5 liters (8). The use of albumin has been shown to significantly reduce the risk of circulatory dysfunction, with a number needed to treat (NNT) of 2, and the risk of hyponatremia, with a NNT of 8 (8). Albumin was not, however, shown to reduce mortality, renal impairment, ascites recurrence, or hospital readmission (8).
Contraindications to paracentesis
There are several (relative) contraindications to consider for paracentesis including an acute abdomen requiring surgery, massive GI bleeding, DIC or other coagulopathy, ileus with bowel distension, overlying abdominal wall cellulitis, and pregnancy (9, 10, 11).
Additionally, one of the most feared concerns is the risk of peri- or post- procedural bleeding. Patients with liver disease commonly have thrombocytopenia and/or coagulopathy. However, routine transfusion of blood products (fresh frozen plasma or platelets) to correct coagulopathy or thrombocytopenia before paracentesis does not seem to mitigate bleeding risk (12). According to national gastroenterology guidelines, coagulation studies are not required before performance of the procedure (12). The incidence of clinically relevant bleeding complications is low (~1%) even if there is an elevated INR at baseline (12). One prospective study of 1100 large-volume paracenteses (99% of which were not ultrasound-guided) found no bleeding complications and no post-procedure transfusions required despite INRs as high as 8.7 and platelet counts as low 19,000/mL (4,11,12).
Complications of paracentesis
Risks of diagnostic or therapeutic paracenteses include bowel perforation, ascitic fluid leak, hemorrhage, and seeding of infection into the peritoneal fluid. Ultrasound guidance decreases the overall risk of complications from ~4.7 to 1.4% (13). Large-volume paracentesis is associated with additional potential complications such as hypotension, hyponatremia, renal impairment, and encephalopathy (8). Additionally, patients should understand that ascites will often recur after removal, and additional paracenteses may be required. All of these should be discussed in depth with the patient before performing the procedure.
The procedure
Finding the fluid pocket:
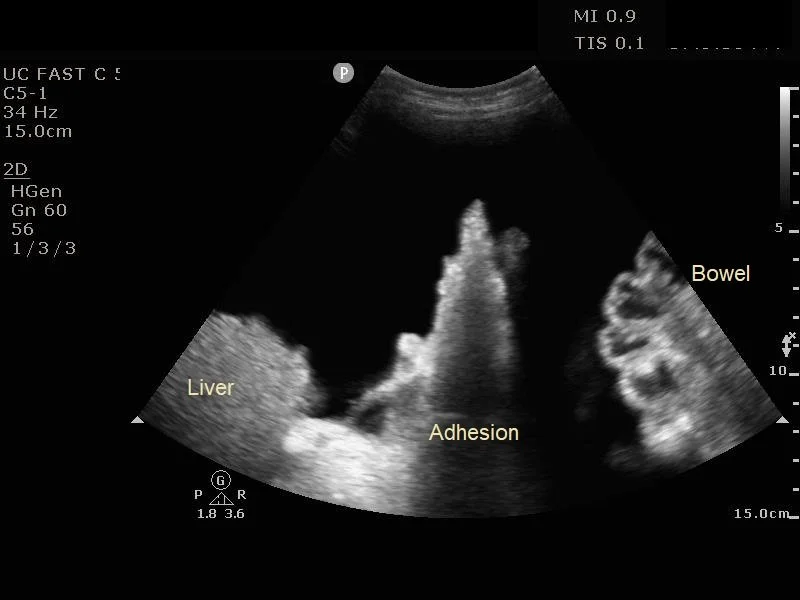
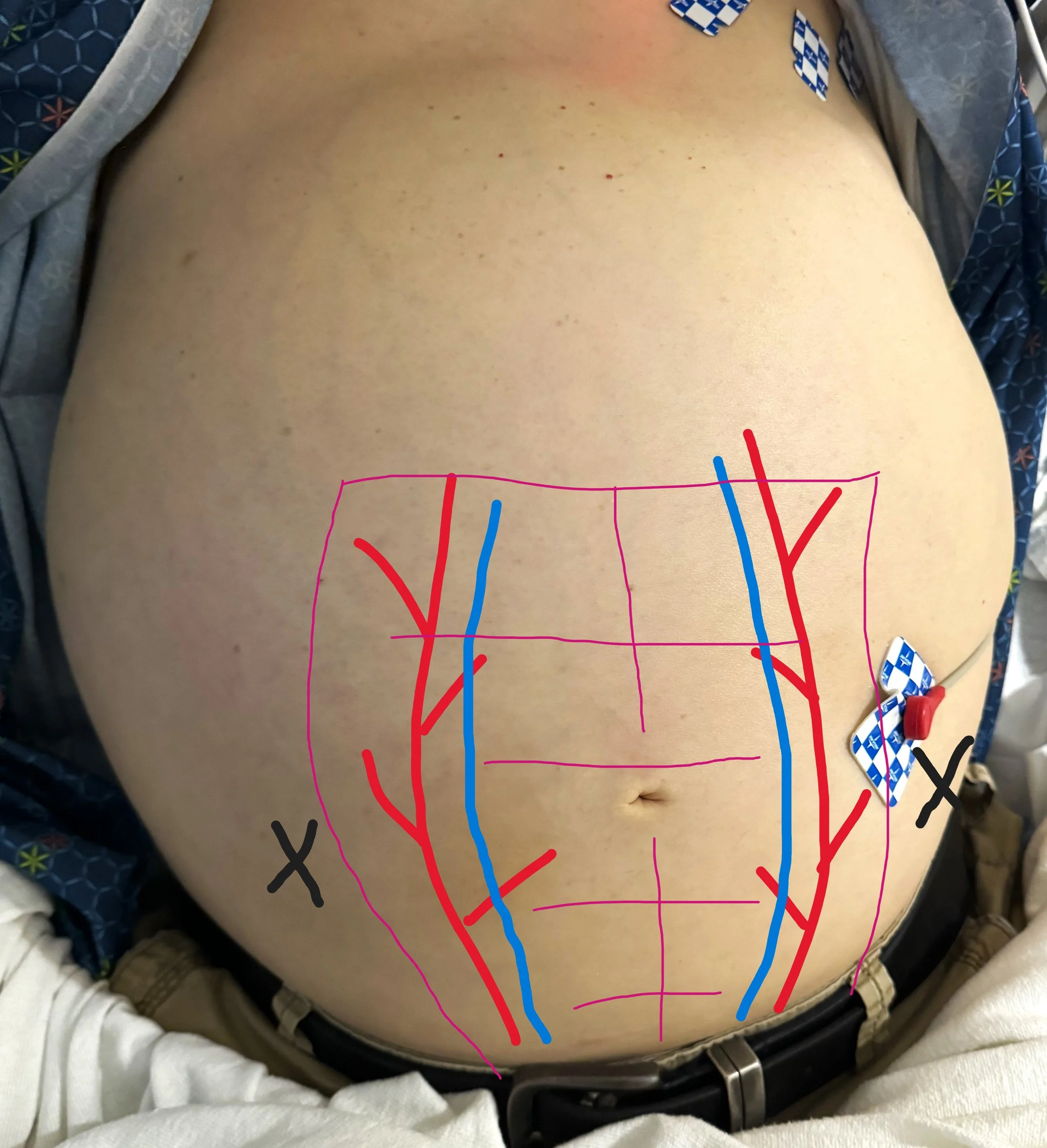
Ultrasound guidance is the standard of care for identifying an appropriate fluid pocket for performance of a paracentesis (14). The ascitic fluid will be shown as a freely mobile anechoic collection in the peritoneal cavity. Although patients can in theory hold fluid pockets anywhere in the abdomen, the most common locations for large pockets are the left and right lower abdominal quadrants lateral to the rectus abdominis muscles (recall that the epigastric vessels lie within the rectus sheath). The left lower quadrant is a good area because this minimizes the potential for liver injury, but the right lower quadrant may also be used if the left lower quadrant has distorted anatomy, such as with prior scarring or ostomy surgery. Sometimes turning the patients on either their right or left side for 10-15 minutes will allow smaller amounts of fluid to accumulate enough to facilitate easier drainage.
For initial evaluation for a fluid pocket, a low-frequency probe such as the curvilinear probe should be used (14). The ideal fluid pocket should be about 3-5 cm in depth between the abdominal wall and viscera / bowel loops. It is important to fan the probe through in multiple angles as bowel loops can appear in all directions. Also evaluate for any adhesions to the abdominal wall, commonly located in the areas of surgical scars. Observe the intended pocket in all phases of respiration as abdominal viscera and bowel loops can change position with respiratory variation. Before committing to a site, using a high frequency linear probe to ensure there are no blood vessels traversing the intended puncture site. This can minimize the risk of inadvertent blood vessel puncture and subsequent hemoperitoneum.
Equipment:
Mask and sterile gloves, gown optional
Sterile drapes
Sterilizing solution (povidone-iodine or chlorhexidine)
Sterile ultrasound probe cover if using real-time guidance
Gauze
Assorted syringes (10, 30, or 60mL)
Small-bore (25 or 27-gauge) needle for anesthetizing the skin and subcutaneous tissue
Medium-bore (18 or 20 -gauge) non-angiocath needles or spinal needles for ascitic fluid aspiration
Local anesthetic (lidocaine)
Purple top EDTA tube for cell count, additional vacutainer tubes for labs as needed
Culture bottles x2 for aerobic and anerobic cultures
Additional supplies for therapeutic or large-volume paracentesis: A source of suction—either vacuum bottles or a setup for wall suction, plastic tubing with luer locks, Catheter-over-needle (available in commercially available paracentesis kits) or angiocath (18- or 16-gauge)
Procedural Steps:
Place patient on cardiac, O2, and blood pressure monitors.
Place the patient in a comfortable supine position with the head of bed slightly elevated and the patient slightly tilted toward left or right side.
Use ultrasound to identify a fluid pocket that is clear of abdominal organs and vessels, at least 3-5 cm deep, and in the left or right lower quadrant. The intended puncture site should avoid any dilated paraumbilical veins (visible on the skin surface) and be lateral to the rectus sheath to avoid the epigastric vessels. A linear probe can be used to evaluate for vasculature. Measure the depth of needle insertion to reach ascitic fluid and maximum depth before encountering viscera or bowel loops.
Mark site of anticipated needle puncture using a needle cap or applying gentle suction on the skin with a syringe
Cleanse the site of expected needle insertion with iodine or chlorhexidine, allow to dry and cover site with sterile drape.
Anesthetize the skin over the target area by raising a wheal, and then aspirate and then inject anesthetic along intended puncture tract until peritoneal fluid is aspirated.
A Z-track technique, in which traction on the skin is used to create a displaced track to the peritoneum while injecting, can minimize the potential for persistent leakage.
Once ascitic fluid is aspirated with the anesthetic syringe and needle, change the syringe with the needle still in place, and then aspirate at least 30-60 cc of fluid into the fresh syringe for laboratory analysis. Alternatively, the anesthetic needle can be removed and a larger bore needle (18-20 gauge) can be inserted with a fresh 60 cc syringe.
Withdraw the needle and cover puncture site with band-aid or gauze and tape.
Inoculate aerobic and anerobic culture bottles with 10 cc of ascitic fluid each with a fresh needle as well as EDTA tube at the bedside to increase diagnostic yield.
Additional Steps for Therapeutic (large volume) Paracentesis:
Insert catheter-over-needle device or angiocath into ascitic fluid as above. Thread catheter or angiocath into peritoneum. Attach plastic tubing to the catheter or angiocath, and connect to suction. Even if the goal is diagnosis, up to 2 L is unlikely to cause complications and may provide significant symptomatic relief.
At completion of fluid removal, withdraw the catheter and cover the insertion site with a dressing. A purse-string suture can be placed to minimize leakage if needed.
Recheck the patient in 30 minutes to identify persistent leakage or an increase in symptoms that would suggest a complication. Patients with large-volume paracentesis should be monitored for low blood pressure for several hours after the procedure.
Troubleshooting
If you are having trouble aspirating fluid, re-verify positioning of the needle using ultrasound with a sterile probe cover. The peritoneum is highly elastic, and occasionally the needle will need to be rotated to pierce through. If flow stops abruptly, the needle tip may have been inadvertently pulled out from the peritoneal cavity, or small bowel or omentum may have occluded the bevel. Gently release suction and re-verify positioning on ultrasound.
Diagnostics
Normal ascitic fluid should appear clear to yellow or straw-colored. Cloudy or turbid fluid can be indicative of infection (15). Blood-tinged or pink fluid can indicate a traumatic tap or be seen with malignancy such as hepatocellular carcinoma.
The initial tests performed on the ascitic fluid should include a gram stain, cell count (with both a total nucleated cell count and polymorphonuclear neutrophils, or PMN count), and culture. If the cause of ascites is unknown, consider sending an ascitic fluid protein and albumin level. The ascitic fluid protein and albumin are measured simultaneously with the serum albumin level to calculate the serum-ascites albumin gradient (SAAG), which help differentiate potential causes of the ascites:
A SAAG greater than 1.1 g/dL (greater or equal to 11 g/L) is 97% sensitive for portal hypertension (16). Pathologies that could lead to portal hypertension include cirrhosis, alcoholic hepatitis, heart failure, massive hepatic metastases, heart failure/pericarditis, Budd-Chiari syndrome, portal vein thrombosis, and idiopathic portal fibrosis (16).
A SAAG less than 1.1 g/dL (less than 11 g/L) suggests extra portal system causes of the ascites leading to loss of protein into the ascitic fluid. This includes patients with peritoneal carcinomatosis, peritoneal tuberculosis, pancreatitis, serositis, and nephrotic syndrome. Other tests to consider include amylase (greater than 1000 U/L suggests pancreatic ascites). Mycobacterial culture should be performed only if tuberculosis is strongly suspected. Other ascitic fluid indices such as lactate and pH offer little to no additional information.
To diagnose SBP, the PMNs or absolute neutrophil count (ANC) in the ascitic fluid must be >250 cells/mm3 (16). In the case of a traumatic tap, the white blood cell count may be falsely elevated. An accepted correction is subtracting 1 neutrophil from the ANC for every 250 RBCs (17).
TREATMENT OF SBP
Early identification and treatment of SBP is imperative. The most commons causative agents include Enterobacter (63%), pneumococcus (15%), enterococci (10%), and anaerobes (<1%) (17). A 3rd-generation cephalosporin such as Cefotaxime 2g IV q8hr or Ceftriaxone 1-2g IV q12-24hr is recommended (17). In the event of beta-lactam allergy, ciprofloxacin 400mg IV q12hr can be used. Renal failure occurs in up to 40% of patients with SBP (18). An albumin infusion (1.5 g/kg with a max dose of 100 g) can reduce the risk of renal failure and in-hospital mortality if given within 6 hours in select patients (18, 19). In patients diagnosed with SBP, an albumin infusion is recommended if the serum creatinine is >1 mg/dL, BUN is > 30 mg/dL or total bilirubin >4 mg/dL) (19).
post by Charlene kotei, md
Dr Kotei is a PGY-1 in Emergency Medicine at the University of Cincinnati.
editing by justine milligan, md and anita goel, md
Dr Milligan is a PGY-4 in Emergency Medicine at the University of Cincinnati with plans to complete an ultrasound fellowship next year, also at the University of Cincinnati.
Dr Goel is an Adjunct Assistant Professor in Emergency Medicine at the University of Cincinnati and an Assistant Editor of TamingtheSRU.
references
Ascites (Nursing) - StatPearls - NCBI Bookshelf, https://www.ncbi.nlm.nih.gov/books/NBK568749/.
Koulaouzidis A, Bhat S, Saeed AA. Spontaneous bacterial peritonitis. World J Gastroenterol. 2009 Mar 7;15(9):1042-9. doi: 10.3748/wjg.15.1042. PMID: 19266595; PMCID: PMC2655193.
Chiejina M, Kudaravalli P, Samant H. Ascites. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470482/
Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. doi:10.1002/hep.20317
Tan, Hiang Keat, et al. “Albumin May Prevent the Morbidity of Paracentesis-Induced Circulatory Dysfunction in Cirrhosis and Refractory Ascites: A Pilot Study.” Digestive Diseases and Sciences, vol. 61, no. 10, 2016, pp. 3084–3092., doi:10.1007/s10620-016-4140-3
Arora V, Vijayaraghavan R, Maiwall R, et al. Paracentesis-Induced Circulatory Dysfunction With Modest-Volume Paracentesis Is Partly Ameliorated by Albumin Infusion in Acute-on-Chronic Liver Failure. Hepatology. 2020;72(3):1043-1055. doi:10.1002/hep.31071
Kulkarni AV, Kumar P, Sharma M, et al. Pathophysiology and Prevention of Paracentesis-induced Circulatory Dysfunction: A Concise Review. J Clin Transl Hepatol. 2020;8(1):42-48. doi:10.14218/JCTH.2019.00048
Albumin for Patients with SBP or Large-Volume Paracentesis. Emergency Medicine. Accessed May 15, 2024. https://emergencymedicine.wustl.edu/items/albumin-for-patients-with-sbp-or-large-volume-paracentesis/#:~:text=Albumin%20reduces%20paracentesis%2Dinduced%20circulatory
Lenz K, Buder R, Kapun L, Voglmayr M. Treatment and management of ascites and hepatorenal syndrome: an update. Therap Adv Gastroenterol. 2015;8(2):83-100. doi:10.1177/1756283X14564673
Runyon BA. Diagnostic and therapeutic abdominal paracentesis. UpToDate. November 8, 2023. Accessed May 15, 2024. https://www.uptodate.com/contents/diagnostic-and-therapeutic-abdominal-paracentesis.
Chapter 65. Paracentesis. In: Reichman EF. eds. Emergency Medicine Procedures, 2e. The McGraw-Hill Companies; 2013. Accessed May 15, 2024. https://accessemergencymedicine.mhmedical.com/content.aspx?bookid=683§ionid=45343705
O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019 Jul;157(1):34-43.e1. doi: 10.1053/j.gastro.2019.03.070. Epub 2019 Apr 12. PMID: 30986390.
Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ. 2012;15(1):1-7. doi: 10.3111/13696998.2011.628723. Epub 2011 Oct 19. PMID: 22011070.
Cho J, Jensen TP, Reierson K, Mathews BK, Bhagra A, Franco-Sadud R, Grikis L, Mader M, Dancel R, Lucas BP; Society of Hospital Medicine Point-of-care Ultrasound Task Force; Soni NJ. Recommendations on the Use of Ultrasound Guidance for Adult Abdominal Paracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E7-E15. doi: 10.12788/jhm.3095. PMID: 30604780; PMCID: PMC8021127.
[Peer-Reviewed, Web Publication] Dyer K, Trinquero P. (2019, July 29). Spontaneous Bacterial Peritonitis. [NUEM Blog. Expert Commentary by Herrine S]. Retrieved from http://www.nuemblog.com/blog/sbp.
Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992 Aug 1;117(3):215-20. doi: 10.7326/0003-4819-117-3-215. PMID: 1616215.
Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000 Jan;32(1):142-53. doi: 10.1016/s0168-8278(00)80201-9. PMID: 10673079.
Gottardi, Andrea De, et al. “Risk of Complications After Abdominal Paracentesis in Cirrhotic Patients: A Prospective Study.” Clinical Gastroenterology and Hepatology, vol. 7, no. 8, 2009, pp. 906–909., doi:10.1016/j.cgh.2009.05.004.
Bernardi, Mauro, et al. “Does the Evidence Support a Survival Benefit of Albumin Infusion in Patients with Cirrhosis Undergoing Large-Volume Paracentesis?” Expert Review of Gastroenterology & Hepatology, 2016, pp. 1–2., doi:10.1080/17474124.2017.1275961.