Pro's and Con's of Procalcitonin
/Procalcitonin was reviewed on Taming the SRU in the context of other biomarkers (ESR, CRP) last year with a grand rounds discussion of its utility in the setting of a febrile 7 week old. As procalcitonin has continued to gain traction in the world of pediatric EM – receiving evaluation as part of proposed protocols for management of febrile infants (1) and for its utility as an aid to diagnosis of Serious Bacterial Infection (2,3) – we turned our attention this year to procalcitonin’s use in adults. Procalcitonin testing has been studied and available for decades (as St. Emlyn’s noted in an update earlier this year), but has not really established itself in widespread use in adults (as EMDocs noted in a sepsis update in 2014). In this blog post, we take the approach of going back to what is known (and is NOT known) about the biochemical basics of this molecule to give context to the sometimes confusing smorgasbord of proposed applications for procalcitonin testing that exists in the literature.
What is procalcitonin anyways?
While knowing what you are testing is no guarantee of easy interpretation (e.g., is this WBC of 12 concerning?), understanding how procalcitonin relates to (patho)physiologic processes provides the mental context required to incorporate testing into clinical decision making – and the vocabulary for the MDM.
For most practitioners, the (patho)physiology of procalcitonin is not easily brought to mind. This isn’t just a lack of publicity, either – procalcitonin’s (patho)physiologic purpose has simply not been well elucidated. This is in contrast to biomarkers like lactate, for which clinicians have internalized a logical chain from clinically apparent physiologic derangements to abnormal test results. Even CRP at least has a clear source in the liver even if its trigger is usually referred to vaguely as “cytokines;” in contrast, the literature vaguely describes the tissue source of procalcitonin in illness as “many different tissues” [4,5].
The biochemistry literature has, however, defined some concrete details about the 116 amino acid polypeptide that is procalcitonin – and these details will ultimately relate directly to clinical applications:
- Procalcitonin is a propeptide that is typically processed into, yes, the hormone calcitonin
- Testing for procalcitonin must target the N-terminal region to distinguish procalcitonin and calcitonin (see Figure A)
- In health, parafollicular C cells in the thyroid produce procalcitonin
- Exact role/function in health independent of precursor role to calcitonin is unclear
- In infection or sepsis (or trauma, surgery, burns, or shock related to non-sepsis causes [4]), a wide range of tissues produce procalcitonin
- Exact role/function in adaptive response to these insults also remains unclear
- Much of the potential utility of procalcitonin as a clinical test relates to two fairly well-characterized aspects of its biochemistry:
- 1) Differential regulation by cytokines
- Because a wide range of tissues (including liver but also many other tissues) produce procalcitonin, its induction must depend on cytokines – including tumor necrosis factor and interleukin-6 [12]. A special role for activation and adherence of monocytic cells in promoting a cytokine milieu resulting in increased procalcitonin has been reported [7].
- Interferon gamma, a cytokine preferentially released during viral infection, suppresses upregulation of procalcitonin [9].
- 2) Kinetics
- Increase in procalcitonin in illness is both rapid and proportionally high relative to its baseline level. The exact rate of increase is variously quoted as significant elevation in 2-4 hours [6,7], 4-6 hours [8] or 6-12 hours after bacterial infection [5]. The variation reflects the authors’ detection method and sense of what degree of increase is required for clinical significance. Regardless, induction time of ~4 hours is significantly shorter than the typical ED patient’s duration of illness at presentation and is faster than CRP [4,7,8]. Peak levels of procalcitonin are reached after 24-48 hours [7].
- Decreases in procalcitonin are accomplished mostly through proteolysis with a small renal clearance contribution [9]. Evaluation of the relative proportion of decrease during treatment is feasible by serial measurements, in part because the decline is fairly rapid in the absence of continued inflammatory stimulation. In the setting of immune system control and/or effective therapy, the expectation is that the procalcitonin level should decline by 30-50% each day [5,9] – consistent with a half-life of approximately 20-26 hours [9].
- 1) Differential regulation by cytokines
How do procalcitonin’s physiological characteristics relate to its clinical utility in the literature?
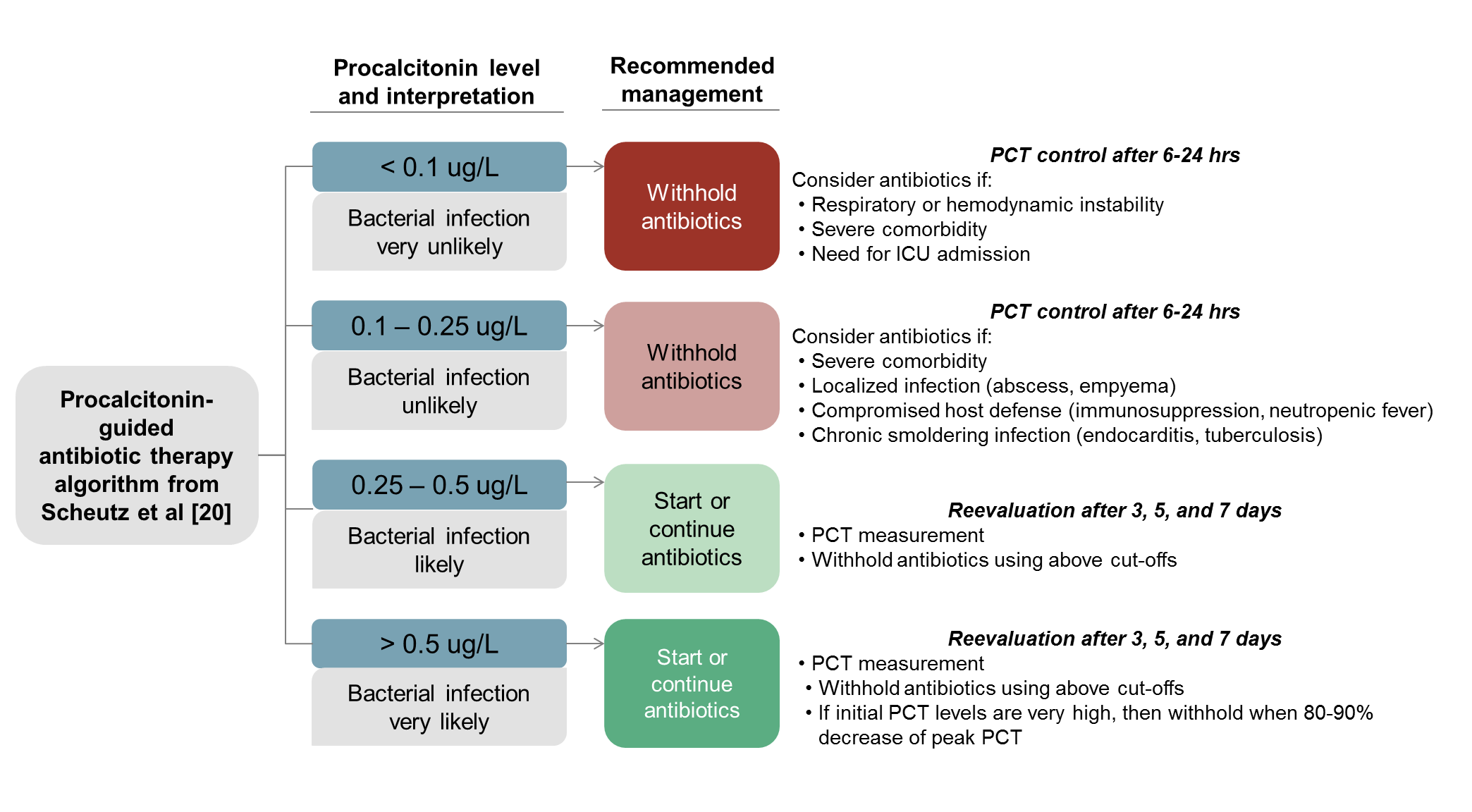
Because interferon gamma is triggered by viruses and suppresses procalcitonin, therefore procalcitonin can differentiate bacterial vs viral etiology, therefore procalcitonin testing can be used to reduce lower respiratory tract infection antibiotic use without affecting mortality (see Figure B)
Because procalcitonin increases rapidly and falls off predictably in the absence of continuing infectious stimuli, data (mostly) support the use of procalcitonin testing to guide antibiotic cessation in ICU patients for acute respiratory tract infection -- but not for all-comers septic patients
Because of the helpful kinetics of procalcitonin, evidence of its decline by 80% in 4 days provides valid prognostic information in general ICU sepsis patients.
For the more curious, here are the details from the literature supporting the headlines above:
Because interferon gamma is triggered by viruses and suppresses procalcitonin, therefore, procalcitonin can differentiate bacterial vs viral etiology, therefore, procalcitonin testing can be used to reduce lower respiratory tract infection antibiotic use without affecting mortality
- A randomized controlled trial, the ProHOSP study, examined outcomes of 1,359 patients in Swiss EDs with acute lower respiratory tract infections (pneumonia, bronchitis, COPD exacerbation) and found procalcitonin-guided antibiotic therapy reduced antibiotic initiation (and duration) without affecting mortality. Based on its data, guidelines were proposed for applying procalcitonin to antibiotic use in RTIs, pictured in Figure B [9, 20].
- A 2017 publication by Wesley Self from Vanderbilt and collaborators provides additional data that procalcitonin can help differentiate bacterial and viral pathogens in pneumonia. In the paper, they analyzed data from the Etiology of Pneumonia in the Community (EPIC) Study, a prospective active surveillance study of 1,735 adults admitted to the hospital with clinical and radiographic evidence of pneumonia. Using a cut-point of 0.1 ng/mL (the first cutpoint from the ProHOSP guidelines), they reported a PPV of 49% and a NPV of 82% -- so using procalcitonin alone as a guide to antibiotic therapy may miss some bacterial infections (even if the ProHOSP data suggests noninferiority for mortality) [12]. In addition, the study population in the Self et al paper is not necessarily externally valid to “judgment call” treatment decisions in the ED since all of its subjects were admitted patients.
Because procalcitonin increases rapidly and falls off predictably in the absence of continuing infectious stimuli, data (mostly) support the use of procalcitonin testing to guide antibiotic cessation in ICU patients – for acute respiratory tract infection but not all-comers septic patients:
- Septic patients in the emergency department often receive broad-spectrum antibiotics early in their course, before source or illness severity are fully evaluated. Once patients move to the ICU or the floor, terminating these antibiotics can be a challenging management decision.
- A Cochrane meta-analysis on acute respiratory tract infections published in 2012 attempted to compare studies where antibiotic therapy had been guided or influenced by procalcitonin to “usual care;” the clinical outcomes (mortality, treatment failure) were similar between the two groups, but procalcitonin guidance was associated with decreased antibiotic duration (median IQR from 8 days to 4 days) [13]. In subgroup analysis of patients who presented to the Emergency Department, no mortality difference was seen and a small, statistically significant reduction in treatment failure was seen with procalcitonin testing; in addition, there were fewer days of antibiotic use (by 3.7 days) and less frequent antibiotic initiation (73% vs 88%) [13].
So the data to this point suggest utility in testing procalcitonin in ED or hospitalized patients, at least with acute respiratory illness. However, what about the septic patient without a definite source, or with a non-respiratory source? Here the data is less compelling and probably does not support procalcitonin testing-guided management in the all-comers septic patient:
- A small, influential study in 2008 supported serial procalcitonin testing to terminate antibiotics [15]. This study of hospitalized ICU patients with severe sepsis or septic shock was limited by its small size (n=79) and the frequent rate of clinician “over-rule” of the study algorithm in the “per protocol” procalcitonin-arm patients (6/31, or 19% of patients) [15].
- A Cochrane meta-analysis covering data through 2015 included 10 RCTs with 1,215 sepsis, severe sepsis, or septic shock patients in which procalcitonin guided decisions in at least one study arm [16]. They rated the data “very low to moderate quality, with insufficient sample power per outcome” and found no definite effect of procalcitonin-guided decisions on mortality, mechanical ventilation, clinical severity, reinfection or duration of antimicrobial therapy of patients with septic conditions [16].
- A Danish study, termed The Procalcitonin and Survival Study (PASS), assessed the outcomes of 1,200 multidisciplinary ICU patients by comparing a procalcitonin-guided arm to a usual care arm. The data showed that a procalcitonin-guided algorithm did not improve mortality and increased ICU days, time on ventilator, and days with impaired GFR [16]. (In case you’re curious, the Cochrane review of procalcitonin in sepsis excluded this study because there were not specific “sepsis” inclusion criteria, although many ICU patients were being treated with antibiotics for diagnosed or suspected sepsis).
Because of the helpful kinetics of procalcitonin, evidence of its decline by 80% in 4 days provides valid prognostic information in general ICU sepsis patients.
- In a prospective, multicenter, observational study of 646 adult ICU patients with sepsis or septic shock (either admitted directly from the ED or from other wards), the authors analyzed 28-day all-cause mortality with an intention-to-diagnose perspective [18]. In this population, if procalcitonin failed to decline by 80% from its initial level on day 4, then the patient had a two-fold higher risk of death (20% vs 10%; p<0.0001); this finding held in a multivariate analysis controlling for other mortality risk factors (hazard ratio 1.97 [95% CI 1.18-3.30]) [18].
As this review of the literature suggests, data support both benefit and harm from procalcitonin testing. Selecting specific clinical scenarios – whether with pediatric patients or acute respiratory tract patients – seems to make the difference. To paraphrase a recent editorial on the matter, procalcitonin testing is the right answer… as long as you ask are asking the right question [19].
References
- Gomez B, Mintegi S, Bressan S, et al. “Validation of the ‘Step-by-Step’ Approach in the Management of Young Febrile Infants.” Pediatrics 2016;138(2):e20154381.
- Milcent K, Faesch S, Gras-Le Guen C, et al. “Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants.” JAMA Pediatr 2016;170(1):62-69.
- Irwin AD, Grant A, Williams R, et al. “Predicting Risk of Serious Bacterial Infections in Febrile Children in the Emergency Department.” Pediatrics 2017;140(2):e20162853.
- Meisner M. “Pathobiochemistry and clinical use of procalcitonin.” Clinica Chemica Acta 2002;323:17-29.
- Manasia A and Narimasu J. “Chapter 7: Biomarkers in Decision Making” In: Oropello JM, Pastores SM, Kvetan V. eds. Critical Care. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=1944§ionid=141465683. Accessed September 14, 2017.
- Becker KL, Snider R, and Nylen ES. “Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations.” Crit Care Med. 2008;36:941-52.
- Meisner M. “Update on procalcitonin measuremnets.” Ann Lab Med 2014;34:263-273.
- van der Does Y, Limper M, Schuit SCE, et al. “Higher diagnostic accuracy and cost-effectiveness using procalcitonin in the treatment of emergency medicine patients with fever (The HiTEMP study): a multicenter randomized study.” BMC Emergency Medicine (2016) 16:17.
- Shaddock EJ. “How and when to use common biomarkers in community-acquired pneumonia.” Pneumonia 2016;8:17.
- Fortunato A. “A new sensitive automated assay for procalcitonin detection.” Practical Lab Med 2016;6:1-7.
- Hoeboer SH, van der Geest PJ, Nieboer D, and Groenweld ABJ. “The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis.” Clin Microbiol Infect 2015;21:474–481
- Self WH, Balk RA, Grijalva CG, et al. “Procalcitonin as a Marker of Etiology in Adults Hospitalized With Community-Acquired Pneumonia.” Clin Inf Dis 2017;65:183-190.
- Schuetz P, Müller B, Christ-Crain M, et al. “Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections.” Cochrane Database of Systematic Reviews 2012, Iss 9. Art. No.: CD007498.
- Westwood ME, Ramaekers BLT, Whiting P, et al. “Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis.” Health Technol Assess 2015;19(96).
- Nobre V, Harbarth S, Graf JD, et al. “Use of Procalcitonin to Shorten Antibiotic Treatment Duration in Septic Patients: A Randomized Trial.” Am J Resp Crit Care Med 2008;177:498-505.
- Andriolo BNG, Andriolo RB, Salomão R, Atallah ÁN. “Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock.” Cochrane Database of Systematic Reviews 2017, Issue 1. Art.No.: CD010959.
- Jensen JU, Hein L, Lundgren B, et al. “Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: A randomized trial.” Crit Care Med 2011;39(9):2048-2058.
- Schuetz P, Birkhahn R, Sherwin R, et al. “Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study.” Crit Care Med 2017;45:781-789.
- Bergin SP and Tsalik EL. “Procalcitonin: The Right Answer but to Which Question?” Clin Inf Dis 2017;65:191-193.
- Schuetz P, Christ-Crain M, Wolbers M, et al. “Procalcitonin guided antibiotic therapy and hospitalization inpatients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial.” BMC Health Svcs Res 2007;7:102.
Written by Bennet Lane, MD, PGY-1
Peer Review/Editing/Posting by Jeffery Hill, MD MEd