Diagnostics and Therapeutics: Managing Pneumothorax
/There are many different types of pneumothorax (PTX), and the management paradigm has shifted in recent years as the research has exploded on this topic. This topic is additionally complicated by the development of multiple diagnostic tools now available for diagnosis as well as variable sizing algorithms used around the world. Institutional resources and specialty services may further dictate the management of PTX. This post aims to broadly cover the types of PTX, the diagnostic modalities available, and the ideal management by PTX type in the Emergency Department.
Types of Pneumothorax
Spontaneous Pneumothorax: Occurs without preceding trauma. It can be further divided into two types: primary--those that occur in generally healthy individuals without underlying lung disease, and secondary--those that occur in individuals with underlying lung disease such as COPD [1].
Primary Spontaneous Pneumothorax: The most common cause of spontaneous PTX and is classically described in tall, thin males or those with unknown connective tissue disorders such as Marfan syndrome, Ehlers Danlos, homocystinuria, or alpha-1 antitrypsin deficiency [1-6]. It is thought to occur due to rupture of small subpleural blebs or bullae, possibly related to increased negative pressure or alveolar stretch that occurs during growth. Smoking is a known risk factor, as is cannabis use [2,5, 7-8].
Secondary Spontaneous Pneumothorax: A less common form of spontaneous PTX that occurs in older patients with underlying lung pathology, particularly COPD, but can also be a complication of emphysema, cystic fibrosis, TB, lung cancer, PJP pneumonia, or interstitial pneumonitis [1, 9-10]. As these patients are typically already at a high risk for poor health outcomes, secondary spontaneous PTX is often more serious in presentation and management more complex [11].
Traumatic Pneumothorax: Occurs secondary to blunt or penetrating trauma and can be further divided into iatrogenic or non-iatrogenic. Overall, this type of PTX is more common, especially as the rate of iatrogenic PTX increases with the increased use of positive pressure ventilation (PPV) and continued use of central venous catheters [12]. One type of traumatic PTX, the tension PTX, requires special consideration given its risk of mortality due to cardiopulmonary compromise [12-13].
Iatrogenic: PTX caused by medical procedures or interventions. Common causes include central venous catheterization (subclavian or internal jugular), lung biopsy, barotrauma from PPV, thoracentesis, bronchoscopy, pacemaker insertion, CPR, and intercostal nerve block [12, 14]. Notably, US guidance decreases the incidence of iatrogenic PTX when used for central venous catheterization [15].
Tension Pneumothorax: Arguably the most serious type of PTX in which an increasing amount of air becomes trapped in the pleural space, causing collapse of the ipsilateral lung and contralateral displacement of mediastinal structures such as the trachea [12]. The subsequent increase in intrathoracic pressure from trapped air decreases venous return to the right heart and can cause hypotension with reflex tachycardia, hypoxia due to poor lung expansion, and ultimately cardiac arrest or death [16]. It is most commonly seen in traumatic PTX however can be a complication of spontaneous PTX under certain conditions and is common in ICU ventilated patients [6]. Other symptoms that may be seen in tension PTX include cyanosis, JVD, and subcutaneous emphysema [14].
Blunt or Penetrating Trauma: Often due to GSW, stab wounds, or blunt chest trauma such as those seen in motor vehicle crashes and falls. Penetrating wounds may allow air to flow into the pleural space from outside the body or from internal disruption of the visceral pleura. Blunt trauma can cause rib fracture or dislocation that may injure the visceral pleura. Alternatively, increased alveolar pressures during blunt thoracic trauma can cause alveolar rupture and subsequent leak of air into the pleural space and resultant bronchopleural fistulas [12, 14].
Occult Pneumothorax: Those discovered incidentally, usually on CT scan, not clinically suspected or seen on chest XR [17].
Diagnosis
Pneumothorax Sizing: There are a number of sizing guidelines that differ based on the country of origin and associated society. The British Thoracic Society has been considered easier to use and determines “large” PTX as >2 cm between the lung margin and the chest wall at the level of a hilum [18]. The American College of Chest Physicians recommends measuring from the lung apex to the cupola but may overestimate the size of apical pneumothoraces; by their definition, a PTX is considered large if this distance is >3 cm [19]. More complicated sizing formulas include the Collins formula [20], the Rhea formula [21], and the Light index [22]. Ultimately, clinical presentation and symptoms are more important than size in determining management [18].
The “35-mm rule”: One retrospective study found that observation is a safe option for the management of pneumothoraces <35 mm on CT (distance between parietal and visceral pleura in the largest air pocket). 89% of patients with PTX under this cutoff were successfully observed. PPV, rib fractures, and decreased GCS were predictive of conservative management failure [23].
Chest XR: Sensitivity 44-47%, Specificity 100% [24-25]. Widely available but has limitations in accurately depicting the size of the PTX. Carries the advantage of being able to detect displaced rib fractures, foreign bodies, mediastinal or diaphragmatic changes [17].
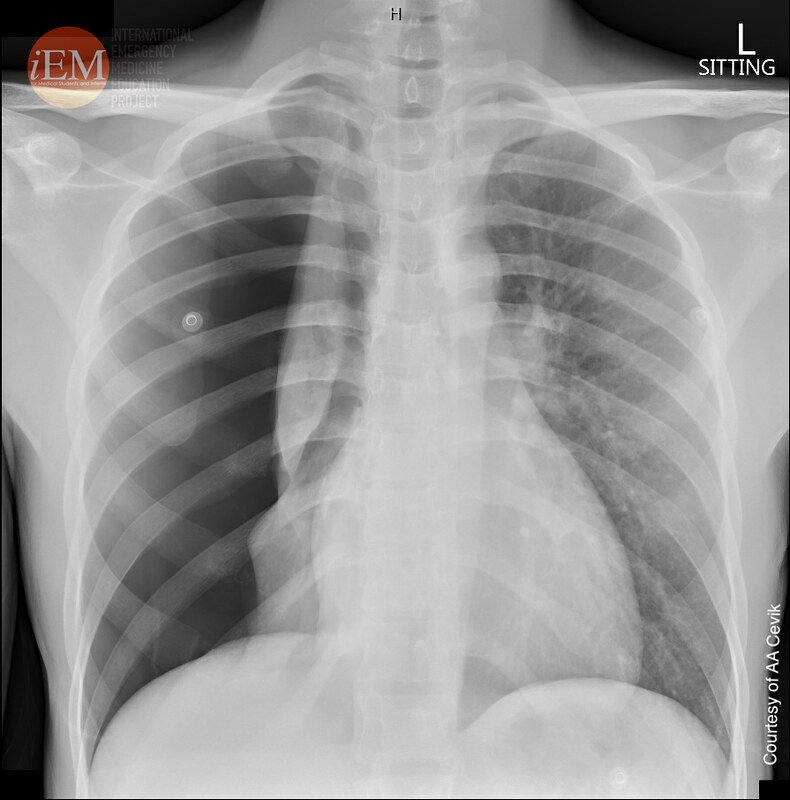
From spontaneous pneumothorax 1 - 18yo male. https://www.flickr.com/photos/iem-student/49098635982
Lung POCUS: Sensitivity 91-98.6%, Specificity 85.5-99% [24-25]. Possibly more useful in trauma patients during e-FAST to determine presence or absence of PTX and less helpful in spontaneous PTX where management is partially based on size, which is difficult to quantify on ultrasound and may be falsely positive in the setting of emphysematous blebs. Stil, absence of lung sliding is the most sensitive finding (see video), whereas a lung point is the most specific [26]. May be considered first line in the future however results may be dependent upon provider training and hospital resources [17].
See this link for a great overview of using lung US to detect PTX
CT Chest: Gold standard for detecting small pneumothoraces and size determination [17]. Is additionally helpful to differentiate bullous lung disease from PTX [18].
Management
Chest Tube: Chest tubes are available in a range of sizes, the smallest of which are ‘pigtail’, or <14 French (Fr), and the largest of which are large-bore catheters up to 40Fr in diameter. Pigtail catheters can be placed using the Seldinger technique whereas large bore chest tubes require a larger incision and guidance of the tube into the pleural space [17]. Pigtail catheters (8.5Fr - 14Fr) have similar efficacy to chest tubes and are associated with faster removal, fewer complications, and shorter hospital LOS [27-31]. If pigtail is unsuccessful, tube thoracostomy should be performed. As this is a painful procedure, the patient should receive adequate anesthesia which may include regional blocks or systemic medications [32].
Observation: A 2010 systematic review of 3 RCTs found no difference in mortality, progression of PTX size, or length of hospital stay between chest tube and observation for stable trauma patients with occult PTX [33]. The 35 mm cutoff has been able to predict successful observation in stable patients with blunt and penetrating chest trauma [23].
Supplemental O2: Patients with O2 saturation <92% should be placed on supplemental O2 [34]. O2 therapy increases the resolution rate of PTX compared to RA, thought to be due to the creation of a diffusion gradient for nitrogen. This benefit may decline after the first 72 hours [35].
Stability: Per the American College of Chest Physicians, a patient with PTX is considered stable if they have a RR < 24, HR 60-120, are normotensive, have an O2 saturation >90% on RA, and are able to speak in complete sentences [19].
Primary Spontaneous Pneumothorax: If they are stable and the patient is not significantly short of breath, it is reasonable to treat with supplemental O2 and observation alone [18-19, 36]. Treating primary spontaneous PTX with invasive procedures does not decrease recurrence rates and increases complications [37]. Patients should be observed for 3-6 hours and have a repeat CXR; if the patient is feeling better and the PTX has not enlarged, they are unlikely to need further intervention [18-19, 34]. If stable but symptomatic, needle aspiration should be performed, ideally using a 14 or 16-Gauge IV catheter or introducer needle to insert an 8Fr or 9Fr catheter in case conversion to pigtail catheter is needed in the setting of unsuccessful aspiration [18, 36]. This is considered successful if there is >2 cm reduction in PTX on the following CXR and the patient’s symptoms have improved. Aspiration has been associated with fewer hospital admissions, shorter hospital LOS, and fewer adverse events when compared to tube thoracostomy, though it did have a lower immediate success rate [38].
Secondary Spontaneous Pneumothorax: For stable patients, supplemental O2 and chest tube/catheter thoracostomy are generally preferred over conservative measures as the risk of aspiration failure, prolonged air leak, and progression to tension PTX are greater in those with underlying lung pathology [39-40]. If the PTX is small and the patient is stable and has minimal symptoms, there may be exceptions to thoracostomy such as observation, supplemental O2, or aspiration--however, the threshold to admit these patients is lower and thus these patients should almost always be admitted [36]. Additionally, outpatient management of secondary spontaneous PTX does not appear to reduce hospital LOS and has a high rate of treatment failure [41]. Any progression of symptoms or increase in PTX size is an indication for intervention. Thoracostomy is preferable to aspiration due to higher failure rates of the latter and a theorized higher rate of persistent air leakage [39, 42-44]. A meta-analysis found that pigtail catheter placement has fewer complication rates than large bore chest tube and may be the first line treatment for secondary spontaneous PTX as well as primary PTX [27]. Those with large air leaks, concomitant empyema or hemothorax, or with barotrauma from mechanical ventilation may benefit instead from large bore chest tube. Ultimately these patients may require VATS or blood patch, chemical pleurodesis, or other procedure to prevent recurrence given the high recurrence rate and likelihood of a life-threatening event.
Traumatic Pneumothorax (Iatrogenic, Blunt or Penetrating Trauma): Large bore chest tubes were traditionally used in case of concomitant hemothorax for theoretically improved drainage and decreased clotting risk [27]. However, recent studies support that pigtail catheters are just as good (if not better) at draining hemothorax and have lower rates of failure as well as reduced pain at the insertion site [17, 45-46].
Tension Pneumothorax: Managed with emergent needle thoracostomy decompression or finger thoracostomy. Classically needle decompression was performed at the second intercostal space in the midclavicular line, however this data was based on older studies and recent increases in our population’s BMI has increased the failure rate at this location. The fifth intercostal space anterior axillary line is now the favored location for needle decompression [17, 47-49]. Finger thoracostomy (aka simple thoracostomy), however, is quicker and easier than tube thoracostomy, and is the only way to definitively know you have reached the inside of the thorax to relieve the tension physiology [50].
See this great TamingtheSRU post of how to perform this procedure! - https://www.tamingthesru.com/blog/acmc/finger-thoracostomy
Occult Pneumothorax: In one study of blunt trauma patients found to have occult pneumothorax, 85% were managed conservatively and only 3.9% of those patients ultimately required chest tube placement [51]. Further studies have identified concomitant subcutaneous air, rib fractures, and pulmonary contusions as predictors of eventual chest tube placement [52-53]. For patients with occult PTX undergoing observation, PPV used in the surgical setting has not been shown to increase the risk of respiratory compromise or mortality, though in one study ⅕ of patients with PTX who were observed ultimately required pleural drainage. Sustained PPV was associated with higher failure of observation [54].
References
Onuki T, Ueda S, Yamaoka M, et al. Primary and Secondary Spontaneous Pneumothorax: Prevalence, Clinical Features, and In-Hospital Mortality. Can Respir J. 2017;2017:6014967. doi:10.1155/2017/6014967
Light RW. Pleural diseases. Dis Mon. 1992;38(5):266-331. doi:10.1016/0011-5029(92)90007-c
Cheng YJ, Chou SH, Kao EL. Familial spontaneous pneumothorax-report of seven cases in two families. Gaoxiong Yi Xue Ke Xue Za Zhi. 1992;8(7):390-394.
Cardy CM, Maskell NA, Handford PA, et al. Familial spontaneous pneumothorax and FBN1 mutations. Am J Respir Crit Care Med. 2004;169(11):1260-1262. doi:10.1164/ajrccm.169.11.967
Melton LJ 3rd, Hepper NG, Offord KP. Influence of height on the risk of spontaneous pneumothorax. Mayo Clin Proc. 1981;56(11):678-682.
Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55(8):666-671. doi:10.1136/thorax.55.8.666
Hedevang Olesen W, Katballe N, Sindby JE, et al. Cannabis increased the risk of primary spontaneous pneumothorax in tobacco smokers: a case-control study. Eur J Cardiothorac Surg. 2017;52(4):679-685. doi:10.1093/ejcts/ezx160
Martinasek MP, McGrogan JB, Maysonet A. A Systematic Review of the Respiratory Effects of Inhalational Marijuana. Respir Care. 2016;61(11):1543-1551. doi:10.4187/respcare.04846
Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev. 2010;19(117):217-219. doi:10.1183/09059180.00005310
Hallifax RJ, Goldacre R, Landray MJ, Rahman NM, Goldacre MJ. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968-2016. JAMA. 2018;320(14):1471-1480. doi:10.1001/jama.2018.14299
Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg. 2013;17(2):247-252. doi:10.1093/icvts/ivt221
Jalota Sahota R, Sayad E. Tension Pneumothorax. In: StatPearls. Treasure Island (FL): StatPearls Publishing; November 28, 2022.
Rojas R, Wasserberger J, Balasubramaniam S. Unsuspected tension pneumothorax as a hidden cause of unsuccessful resuscitation. Ann Emerg Med. 1983;12(6):411-412. doi:10.1016/s0196-0644(83)80502-2
Sharma A, Jindal P. Principles of diagnosis and management of traumatic pneumothorax. J Emerg Trauma Shock. 2008;1(1):34-41. doi:10.4103/0974-2700.41789
Vinson DR, Ballard DW, Hance LG, et al. Pneumothorax is a rare complication of thoracic central venous catheterization in community EDs. Am J Emerg Med. 2015;33(1):60-66. doi:10.1016/j.ajem.2014.10.020
Nelson D, Porta C, Satterly S, et al. Physiology and cardiovascular effect of severe tension pneumothorax in a porcine model. J Surg Res. 2013;184(1):450-457. doi:10.1016/j.jss.2013.05.057
Tran J, Haussner W, Shah K. Traumatic Pneumothorax: A Review of Current Diagnostic Practices And Evolving Management. J Emerg Med. 2021;61(5):517-528. doi:10.1016/j.jemermed.2021.07.006
MacDuff A, Arnold A, Harvey J; BTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii18-ii31. doi:10.1136/thx.2010.136986
Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590-602. doi:10.1378/chest.119.2.590
Collins CD, Lopez A, Mathie A, Wood V, Jackson JE, Roddie ME. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol. 1995;165(5):1127-1130. doi:10.2214/ajr.165.5.7572489
Rhea JT, DeLuca SA, Greene RE. Determining the size of pneumothorax in the upright patient. Radiology. 1982;144(4):733-736. doi:10.1148/radiology.144.4.7111716
Light RW. Management of spontaneous pneumothorax. Am Rev Respir Dis. 1993;148(1):245-248. doi:10.1164/ajrccm/148.1.245
Bou Zein Eddine S, Boyle KA, Dodgion CM, et al. Observing pneumothoraces: The 35-millimeter rule is safe for both blunt and penetrating chest trauma. J Trauma Acute Care Surg. 2019;86(4):557-564. doi:10.1097/TA.0000000000002192
Chan KK, Joo DA, McRae AD, et al. Chest ultrasonography versus supine chest radiography for diagnosis of pneumothorax in trauma patients in the emergency department. Cochrane Database Syst Rev. 2020;7(7):CD013031. Published 2020 Jul 23. doi:10.1002/14651858.CD013031.pub2
Dahmarde H, Parooie F, Salarzaei M. Accuracy of Ultrasound in Diagnosis of Pneumothorax: A Comparison between Neonates and Adults-A Systematic Review and Meta-Analysis. Can Respir J. 2019;2019:5271982. Published 2019 Dec 3. doi:10.1155/2019/5271982
Lichtenstein D, Mezière G, Biderman P, Gepner A. The "lung point": an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. doi:10.1007/s001340000627
Chang SH, Kang YN, Chiu HY, Chiu YH. A Systematic Review and Meta-Analysis Comparing Pigtail Catheter and Chest Tube as the Initial Treatment for Pneumothorax. Chest. 2018;153(5):1201-1212. doi:10.1016/j.chest.2018.01.048
Benton IJ, Benfield GF. Comparison of a large and small-calibre tube drain for managing spontaneous pneumothoraces. Respir Med. 2009;103(10):1436-1440. doi:10.1016/j.rmed.2009.04.022
Tsai WK, Chen W, Lee JC, et al. Pigtail catheters vs large-bore chest tubes for management of secondary spontaneous pneumothoraces in adults. Am J Emerg Med. 2006;24(7):795-800. doi:10.1016/j.ajem.2006.04.006
Iepsen UW, Ringbæk T. Small-bore chest tubes seem to perform better than larger tubes in treatment of spontaneous pneumothorax. Dan Med J. 2013;60(6):A4644.
Voisin F, Sohier L, Rochas Y, et al. Ambulatory management of large spontaneous pneumothorax with pigtail catheters. Ann Emerg Med. 2014;64(3):222-228. doi:10.1016/j.annemergmed.2013.12.017
Lin J, Hoffman T, Badashova K, Motov S, Haines L. Serratus Anterior Plane Block in the Emergency Department: A Case Series. Clin Pract Cases Emerg Med. 2020;4(1):21-25. Published 2020 Jan 21. doi:10.5811/cpcem.2019.11.44946
Yadav K, Jalili M, Zehtabchi S. Management of traumatic occult pneumothorax. Resuscitation. 2010;81(9):1063-1068. doi:10.1016/j.resuscitation.2010.04.030
Brown SGA, Ball EL, Perrin K, et al. Conservative versus Interventional Treatment for Spontaneous Pneumothorax. N Engl J Med. 2020;382(5):405-415. doi:10.1056/NEJMoa1910775
Park CB, Moon MH, Jeon HW, et al. Does oxygen therapy increase the resolution rate of primary spontaneous pneumothorax?. J Thorac Dis. 2017;9(12):5239-5243. doi:10.21037/jtd.2017.10.149
Gottlieb M, Long B. Managing Spontaneous Pneumothorax. Ann Emerg Med. 2023;81(5):568-576. doi:10.1016/j.annemergmed.2022.08.447
Liu WL, Lv K, Deng HS, Hong QC. Comparison of efficiency and safety of conservative versus interventional management for primary spontaneous pneumothorax: A meta-analysis. Am J Emerg Med. 2021;45:352-357. doi:10.1016/j.ajem.2020.08.092
Carson-Chahhoud KV, Wakai A, van Agteren JE, et al. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database Syst Rev. 2017;9(9):CD004479. Published 2017 Sep 7. doi:10.1002/14651858.CD004479.pub3
Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society Guideline for pleural disease. Thorax. 2023;78(Suppl 3):s1-s42. doi:10.1136/thorax-2022-219784
Chee CB, Abisheganaden J, Yeo JK, et al. Persistent air-leak in spontaneous pneumothorax--clinical course and outcome. Respir Med. 1998;92(5):757-761. doi:10.1016/s0954-6111(98)90008-7
Walker SP, Keenan E, Bintcliffe O, et al. Ambulatory management of secondary spontaneous pneumothorax: a randomised controlled trial. Eur Respir J. 2021;57(6):2003375. Published 2021 Jun 24. doi:10.1183/13993003.03375-2020
Light RW. Pleural controversy: optimal chest tube size for drainage. Respirology. 2011;16(2):244-248. doi:10.1111/j.1440-1843.2010.01913.x
Andrivet P, Djedaini K, Teboul JL, Brochard L, Dreyfuss D. Spontaneous pneumothorax. Comparison of thoracic drainage vs immediate or delayed needle aspiration. Chest. 1995;108(2):335-339. doi:10.1378/chest.108.2.335
Kiely DG, Ansari S, Davey WA, Mahadevan V, Taylor GJ, Seaton D. Bedside tracer gas technique accurately predicts outcome in aspiration of spontaneous pneumothorax. Thorax. 2001;56(8):617-621. doi:10.1136/thorax.56.8.617
Kulvatunyou N, Erickson L, Vijayasekaran A, et al. Randomized clinical trial of pigtail catheter versus chest tube in injured patients with uncomplicated traumatic pneumothorax. Br J Surg. 2014;101(2):17-22. doi:10.1002/bjs.9377
Inaba K, Lustenberger T, Recinos G, et al. Does size matter? A prospective analysis of 28-32 versus 36-40 French chest tube size in trauma. J Trauma Acute Care Surg. 2012;72(2):422-427. doi:10.1097/TA.0b013e3182452444
Inaba K, Branco BC, Eckstein M, et al. Optimal positioning for emergent needle thoracostomy: a cadaver-based study. J Trauma. 2011;71(5):1099-1103. doi:10.1097/TA.0b013e31822d9618
Inaba K, Karamanos E, Skiada D, et al. Cadaveric comparison of the optimal site for needle decompression of tension pneumothorax by prehospital care providers. J Trauma Acute Care Surg. 2015;79(6):1044-1048. doi:10.1097/TA.0000000000000849
Laan DV, Vu TD, Thiels CA, et al. Chest wall thickness and decompression failure: A systematic review and meta-analysis comparing anatomic locations in needle thoracostomy. Injury. 2016;47(4):797-804. doi:10.1016/j.injury.2015.11.045
Massarutti D, Trillò G, Berlot G, et al. Simple thoracostomy in prehospital trauma management is safe and effective: a 2-year experience by helicopter emergency medical crews. Eur J Emerg Med. 2006;13(5):276-280. doi:10.1097/00063110-200610000-00006
Mahmood I, Younis B, Ahmed K, et al. Occult Pneumothorax in Patients Presenting with Blunt Chest Trauma: An Observational Analysis. Qatar Med J. 2020;2020(1):10. Published 2020 Mar 16. doi:10.5339/qmj.2020.10
Ball CG, Kirkpatrick AW, Laupland KB, et al. Incidence, risk factors, and outcomes for occult pneumothoraces in victims of major trauma. J Trauma. 2005;59(4):917-925. doi:10.1097/01.ta.0000174663.46453.86
Nguyen L, Bowlds S, Juan D, et al. Predictors of Pneumothorax (POP) Score: A Statistical Model for Identifying Occult Pneumothoraces after Blunt Chest Trauma That Will Require Intervention. Am Surg. 2019;85(9):e470-e472.
Kirkpatrick AW, Rizoli S, Ouellet JF, et al. Occult pneumothoraces in critical care: a prospective multicenter randomized controlled trial of pleural drainage for mechanically ventilated trauma patients with occult pneumothoraces.J Trauma Acute Care Surg. 2013;74(3):747-755. doi:10.1097/TA.0b013e3182827158
Post by Erin Vaughn, MD
Dr. Vaughn is a PGY-1 in Emergency Medicine at the University of Cincinnati
Editing by Ryan LaFollette, MD
Dr. LaFollette is an Associate Professor in Emergency Medicine at the University of Cincinnati and co-editor of TamingtheSRU
Peer Review and Editing by Jeffery Hill, MD MEd, Associate Professor, University of Cincinnati Department of Emergency Medicine
Cite As
Vaughan, E. LaFollette, R. Hill, J. Diagnostics and Therapeutics: Managing Pneumothorax. TamingtheSRU. www.tamingthesru.com/diagnostics/pneumothorax. 10/2/2023