Annals of B-Pod: Intra-Aortic Balloon Pump
/Intra-Aortic Balloon Pump Basics
The intra-aortic balloon pump (IABP), first developed in the 1960s, is one of the most widely-used cardiac assist devices. Placed in critically-ill patients with cardiogenic shock, it increases coronary blood flow and decreases afterload. Patients with IABPs are frequently transferred to tertiary referral hospitals via helicopter emergency medical services (HEMS) transport. As such, prehospital and ED providers must become comfortable with the management of these patients and be aware of complications from these devices. Two recent Air Care patients highlight this need.
Case 1
The patient is a female in her 60s who presented to an outside hospital with chest pain and was found to have an anterior ST elevation MI (STEMI). She underwent stenting of the left anterior descending (LAD) coronary artery, but continued to have ongoing severe chest pain. Repeat coronary angiography revealed no evidence of in-stent thrombosis, but was notable for poor flow to additional coronary arteries. As such, an IABP was placed secondary to persistent unstable angina. On Air Care arrival, she was alert and hemodynamically stable with a heart rate in the 80s and a blood pressure 120s over 70s.
The patient was transitioned to Air Care’s IABP portable console while still on the cath lab table. The IABP was set to ECG trigger, 1:1 assist ratio and had good capture on the monitor. The patient’s diastolic pressure was augmented into the 130s. She tolerated transport well and was taken to the Cardiovascular Intensive Care Unit without any hemodynamic instability. The IABP was removed within 24 hours, as she remained hemodynamically stable and reported improvement in chest pain.
Case 2
The patient is a male in his late 40s who presented to an outside hospital five days after an inferior STEMI. Of note, coronary angiography at that time revealed chronic total occlusion of the right coronary artery. However, revascularization was unsuccessful, and he was treated with medical management. Upon repeat presentation, the patient reported continuing shortness of breath and chest pain since being discharged. A bedside echocardiogram revealed severe mitral regurgitation and thrombus in the left ventricle.
The patient was taken back to the cath lab and found to have papillary muscle rupture. While in the cath lab, the patient suffered a PEA arrest with subsequent return of spontaneous circulation (ROSC). An IABP was then placed and Air Care was called for emergent transport to our hospital for operative repair of the valve. On Air Care arrival, vitals were notable for a blood pressure of 90s/50s with a heart rate in the 90s. The IABP was set to pressure trigger at 1:1 assist ratio. Exam was notable for a holosystolic murmur, thready distal pulses, diffuse crackles in all lung fields with pink, frothy ETT secretions and significant JVD.
As the patient was being loaded into the aircraft, he became bradycardic to the 30s. Push-dose epinephrine was administered with improvement in his heart rate. However, once in the aircraft, the patient went into ventricular tachycardia with thready pulses before quickly proceeding into asystole. CPR and ACLS were initiated during take-off and continued during the flight. Resuscitative efforts continued on arrival in the ED for an additional 25 minutes without ROSC. Cardiology and Cardiothoracic Surgery evaluated the patient at bedside in the ED and agreed with terminating efforts based on down time and prognosis.
Discussion
These cases illustrate two extremes of patients that prehospital HEMS and Emergency Medicine providers may encounter with IABPs. These assist devices are indicated for use in patients with acute cardiogenic shock, failure to wean off cardiopulmonary bypass after cardiothoracic surgery, refractory unstable angina, or ventricular arrhythmias. Although initially developed for use in the perioperative period for coronary artery bypass grafting (CABG), there is increasing use for those patients with acute cardiogenic shock. The pumps are contraindicated in those with aortic dissection or severe aortic regurgitation, and are cautiously used in those with severe peripheral vascular disease or coagulopathies.[1]
The IABP is composed of a catheter with a distal cylindrical balloon sized by patient height. This is connected to a console with a helium tank used to inflate the balloon. Helium is used as it is an inert gas which is easily absorbed into blood in the rare event of balloon rupture. The console is also connected to ECG leads and an arterial pressure line, which are used to trigger the inflation of the balloon at the appropriate time within the cardiac cycle and monitor hemodynamic response. Newer models augment the traditional arterial line with a fiberoptic cable built into the balloon catheter itself, allowing for auto-calibration of timing.
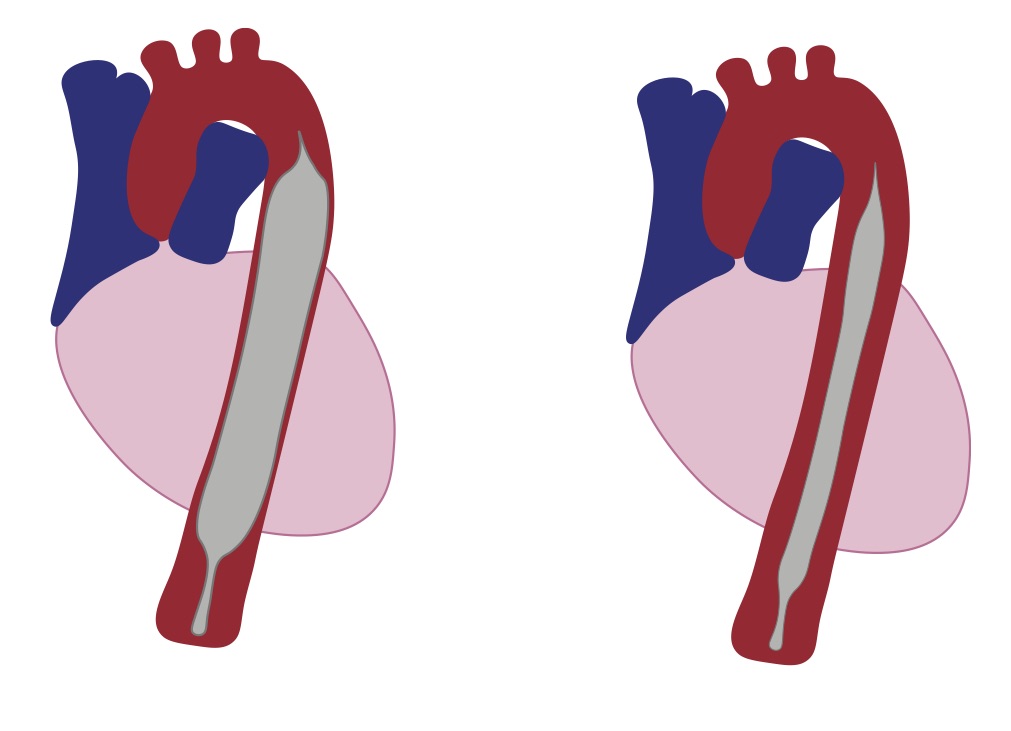
The purpose of the IABP is to increase coronary perfusion and myocardial oxygen supply while decreasing myocardial oxygen demand by several mechanisms. A balloon sits in the proximal descending aorta, approximately 1 cm distal to the left subclavian artery, and is triggered to inflate and deflate at different phases of the cardiac cycle (Figure 1).
The balloon is triggered to inflate in early diastole, which causes an increase in the early diastolic blood pressure (DBP), subsequently increasing coronary perfusion. Deflation occurs in early systole, just before the aortic valve opens. This creates a vacuum effect, reducing cardiac afterload by decreasing aortic end-DBP and decreasing myocardial demand. These physiologic alterations serve to benefit both those in acute cardiogenic shock and those with acute myocardial infarction.
As discussed previously, the balloon is triggered to inflate and deflate based on the cardiac cycle. For most patients, this is done by placing the balloon pump in the ECG trigger mode, which is based off the R-wave, marking the beginning of systole. Newer models automatically calculate the timing of the cardiac cycle and will then inflate at the appropriate time, which is mid T-wave or early diastole.
For all models, there are other trigger modes including pressure trigger and pacer trigger. Pressure mode may be used for backup when ECG leads are not reading, there is significant artifact or the patient is in arrest. The trigger in this case is the systolic upstroke of the arterial waveform. Pacer mode is used for patients with 100% AV/V pacing where there are no reliable R-waves to trigger the ECG mode. ECG mode will work, however, for the majority of paced patients that have good capture. Regardless of the trigger mode, the arterial waveform is used to determine if the timing with the cardiac cycle is accurate.
In addition to the trigger mode, the operator must also set the assist ratio. Assist ratio is the number of assisted cycles to the number of intrinsic beats. The most common is 1:1 and is efficient up to 120 beats per minute. The 1:2 and 1:3 ratios are most often used for weaning from the IABP in the postoperative period or in extremely tachycardic patients.
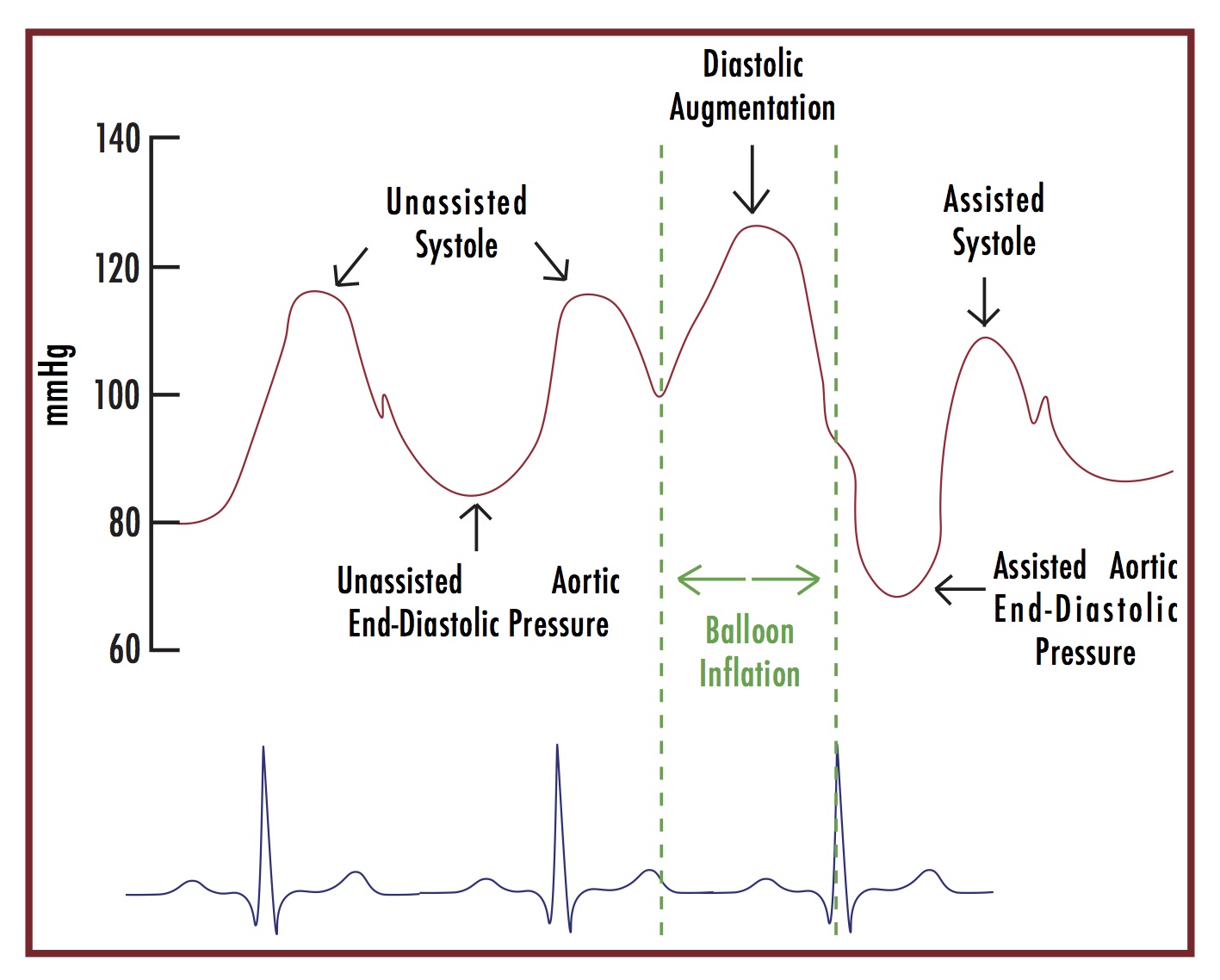
Figure 2 illustrates the optimized arterial pressure waveform with IABP augmentation.[2] The goal is to have appropriate timing of inflation and deflation with the cardiac cycle in order to most effectively improve hemodynamics. There are several free resources available with scenarios of poorly timed waveforms and their solutions that may be helpful.[3,6] The newer models, including the Maquet Cardiosave HybridTM that are carried on Air Care, are equipped with an “auto-mode” that automatically fills the balloon, performs calibration using fiber-optic cable, selects the most appropriate lead and trigger, and sets the appropriate inflation timing.
While Emergency Medicine physicians must have an understanding of which patients are candidates for urgent IABP placement and how these devices function, it is essential for those involved in prehospital care and those at quaternary referral centers to know the basics of managing a patient who already has one of these devices in place. The largest study to date of IABP complications found that the most common complications included limb ischemia (2.9%), bleeding from the access site or from aorto-iliac dissection (2.4%), balloon rupture (1.0%) and death attributable to balloon pump (0.05%).[5]
Prior to transport, the HEMS provider should always perform a neurovascular exam and check the access site for hematoma or active bleeding. Any report of sudden change in the quality or degree of pain should raise concern for dissection and the vascular exam should be repeated immediately. If the provider notices blood in the helium line, this is representative of a balloon rupture, which can be life-threatening due to exsanguination through the line. Clamping the line with a Kelly clamp or hemostat can temporize this complication.
Another issue that arises in the emergent care of these patients is represented by the second case above. There are a few pertinent points to be aware of if the patient that experiences cardiac arrest. First, CPR should be performed as usual in these patients without fear of damaging the balloon. Secondly, when set to “auto-mode,” the newer machines will automatically switch between ECG and pressure trigger mode depending on the signal quality. This prevents the machine from sensing R-waves from artifact and impeding systemic and cardiac perfusion when compressions are being done. The pressure mode will sense the increased pressure in the aorta from external compressions and time the inflation cycle to match and improve coronary perfusion.
Additionally, defibrillation may be used if the patient is in a shockable rhythm. The machine is grounded, but everyone should stand clear of the machine to prevent being shocked if it is not plugged into a wall outlet. Finally, the pump should not be placed in “standby mode” for greater than 30 minutes due to the risk of thrombosis on the balloon itself, as these patients are not typically systemically anticoagulated while the IABP is in place.
For the prehospital or ED provider, it is important to understand the basic workings of balloon pumps and their complications. Ultimately, care of the patient with an IABP should proceed as usual when treating the underlying condition necessitating the pump.
See more about the logistics of IABPs on Air Care here.
Authored by Collins Harrison, MD Posted by Grace Lagasse, MD
References
- IABP Guide. National Heart Centre Singapore. https://www.nhcs.com.sg/educationandtraining/CMEcollaterals/Documents/(AaronWong)IABP–IndicationforandbeyondCardiogenicshock.pdf
- Principles of intra-aortic balloon pump counterpulsation. Contin Educ Anaesth Crit Care Pain (2009) 9 (1): 24-28. doi: 10.1093/bjaceaccp/mkn051
- Intra-aortic Balloon Pump Trouble Shooting. http://lifeinthefastlane.com/cardiovascular-curveball-007/
- Maquet White Paper on Conterpulsion Theory. http://www.maquet.com/globalassets/downloads/products/shared-cardiosave/us/ca_cardiosave-cs300-theory-and-technique_clinical-manual_9798-us-rev-b_eng.pdf?lang=en-US&src=/us/products/cardiosave-iabp-hybrid/?ccid=174)
- Ferguson J, III, Cohen M, Freedman R, Jr, et al. The current practice of intra-aortic balloon counterpulsation: results from the Benchmark Registry. J Am Coll Cardiol. 2001;38(5):1456-1462. doi:10.1016/S0735-1097(01)01553-4.