Annals of B-Pod: Making sense of Pneumonia Acronyms
/The patient is a middle aged male with a history of diet-controlled diabetes who presented to the Emergency Department from work with chest pain, cough and shortness of breath. He describes the chest pain as anterior, non-radiating and throbbing, and states that it started early this morning. The patient states later in the day he noted the development of diaphoresis and shortness of breath, which he described as worse with exertion. He also reported a non-productive cough since waking this morning. He took ibuprofen at home without any relief. He denied fevers, nausea, vomiting, abdominal pain, changes in vision, syncope, and palpitations. He denies tobacco use.
Vitals: T 36.1 HR 93 BP 158/89 RR 16 SpO296% on RA
Physical Exam: The patient appeared in no acute distress. His lung sounds were clear to auscultation bilaterally with good air entry, and was without wheezes, rales, rhonchi or any signs of respiratory distress. His cardiovascular exam revealed a regular rate and rhythm without murmurs or rubs. He was noted to have tenderness to palpation over his anterior his left chest wall and epigastrium. His abdominal exam was benign, and his skin exam did not reveal any rashes. His neurologic exam revealed no gross deficits.
Diagnostics
WBC 12.6 Na+ 135 K+ 3.6 BUN 12 troponin < 0.04 x 3
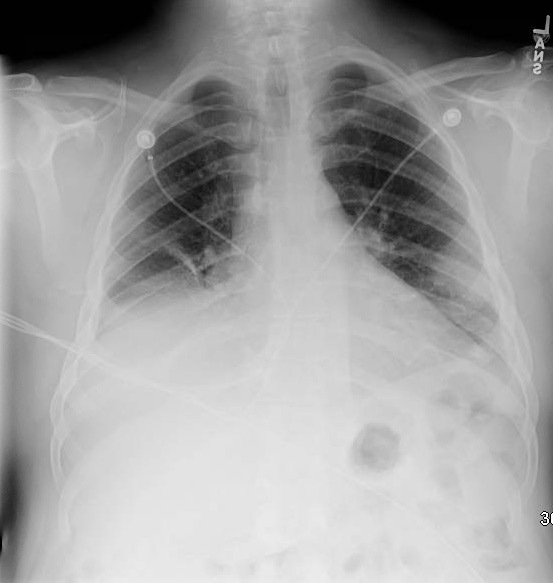
CXR: New opacification of the right middle lobe with findings suggesting volume expansion, as well as partial obscuring of the elevated right hemidiaphragm. Image 1.
CT Chest: Elevated right hemidiaphragm with complete collapse of right middle lobe, as well as a patchy infiltrate left lower lobe with surrounding groundglass opacities, consistent with pneumonia
Hospital Course
The patient presented to the ED with chest pain, a new cough, diaphoresis and dyspnea on exertion. The initial work up revealed normal labs with the exception of a mild leukocytosis and normal serial troponins. An EKG was also normal.
However, his initial chest x-ray showed collapse of the right middle lobe of the lung. A CT of the chest was pursued for further characterization of this new x-ray finding, which revealed collapse of the right middle lobe and infiltrate in the right lower lobe, consistent with pneumonia. He was otherwise well-appearing and had no oxygen requirement. His CURB-65 score was 0. This patient was diagnosed with community-acquired pneumonia and was prescribed five days of levofloxacin. He was discharged and did not return to the ED.
Pneumonia
Pneumonia is defined by the 2005 Infectious Diseases Society of America guidelines as “the presence of a new lung infiltrate plus clinical evidence that the infiltrate is of an infectious origin, which includes fever, purulent sputum, leukocytosis, and decline of oxygenation.”1 Affecting millions of Americans each year, the diagnosis carries significant morbidity and mortality, especially if the diagnosis is delayed. When combined with flu, pneumonia is the 7th leading cause of death worldwide and responsible for 1.2 million hospitalizations in the United States annually.[2] As pneumonia represents a significant disease burden, IDSA and the American Thoracic Society (ATS) combined to formulate guidelines for the diagnosis and treatment of pneumonia to improve clinical outcomes. Self et al estimated that 2.2% of all ED visits are for pneumonia, representing 7-8 ED visits per 1000 persons annually.[2] As, such, timely identification of pneumonia and appropriate antibiotic choice is of critical importance for the Emergency Physician.
CAP? HAP? VAP? What does it all mean?
Traditionally, the discussion of pneumonia management has been delineated into two discrete patient populations with unique risk factors for specific pathogens, leading to the disease categories of community-acquired pneumonia (CAP) and healthcare-associated pneumonia (HCAP). However, these categories have recently changed, as have the recommendations for management of different patient populations diagnosed with pneumonia.
First suggested as a separate clinical entity in the 2005 Infectious Diseases Society ofAmerica and the American Thoracic Society (IDSA-ATS) guidelines, the term HCAP was created to identify patients at risk for multi-drug resistant organisms (MDRO) and treat them with appropriate empiric antibiotics. Risk factors for HCAP included: hospitalization greater than 48 hours in the last 90 days; extended-care facility residents; patients undergoing home infusion therapy, home wound care or chronic dialysis within one month; and patients with a family member previously treated for a MDRO.[2]
However, a recent large meta-analysis[4] showed that these defined HCAP criteria did not predict which patients would ultimately be diagnosed with an MDRO. In fact, many of the HCAP patients were ultimately identified to have pathogens classically associated with community-acquired pneumonia, and many of the patients who ended up having MDRO organisms initially presented from the community.
Thus, in a recently-released set of IDSA-ATS guidelines focusing on nosocomial pneumonia, the concept of HCAP was abandoned in favor of the terms hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).[5] A new set of CAP guidelines are expected to be released this year, and are anticipated to include guidance about patients presenting from the community who are at risk for MDROs.
Overall, the IDSA recommends that for all patients presenting with concern for pneumonia, empiric antibiotic choice and disease management should be based upon the individual patient's presentation, the severity of their illness, their unique risk factors for drug-resident pathogens, and the local antibiogram and outcomes.
Community-acquired pneumonia
Community-acquired pneumonia (CAP) is defined as pneumonia acquired from the community, as opposed to a nosocomial infection. It is a diagnosis made by clinical features such as cough, fever, sputum production, and chest pain, as well as physical exam findings such as rales or bronchial breath sounds.
However, like with the above patient, a new infiltrate on chest x-ray is considered the gold standard for diagnosis.[2] Cross-sectional imaging can also be useful when plain films are equivocal. While most guidelines recommend tailoring antibiotic choice to an identified pathogen, there is no consensus as to how best to obtain a microbiologic specimen, with recommendations ranging from relying on local antibiograms to obtaining sputum cultures, blood cultures and urinary antigens.[6] In general, patients who are being admitted to the hospital for CAP should at the minimum have a sputum culture obtained if feasible, but this is optional for patients managed in the outpatient setting.
Community-acquired pneumonia can be caused by a variety of pathogens, including viral etiologies. Of bacterial causes, Streptococcus pneumoniae is the most common, but may also include such other typical organisms like Haemophilus influenzae, Staphylococcus aureus, or group A strep. Classically "atypical" organisms include Legionella species, Mycobacterium tuberculosis or C. pneumoniae. Viruses include influenza, RSV, parainfluenza and adenoviruses.[6]
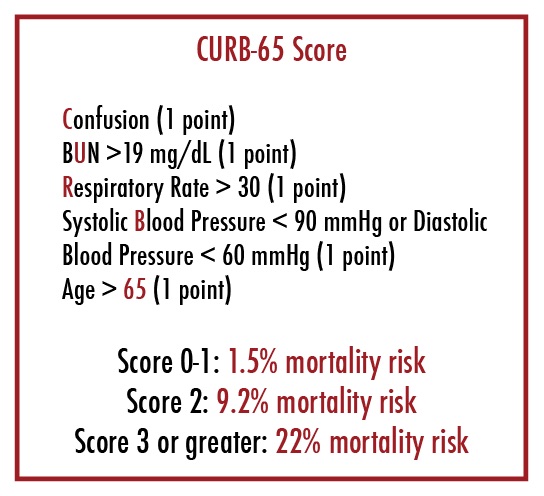
While many patients presenting with pneumonia from the community may be appropriate for outpatient management, some may require hospital admission and may even be critically ill. Multiple scoring systems exist to predict CAP severity and aid in disposition planning. CURB-65 and the pneumonia severity index (PSI) are two such tools. The CURB-65 score (Figure 1) uses five prognostic factors–confusion, BUN, elevated respiratory rate, blood pressure and age greater than 65–to predict mortality. In general, CURB-65 scores greater than 2 warrant hospital admission, and those with scores greater than 3 likely need ICU-level care. [7,8]
For patients who can be safely treated in the outpatient setting, the IDSA recommends that these patients receive a first dose of antibiotics in the ED. Treatment should last a minimum of 5 days. In terms of antibiotic choice, the guidelines recommend adhering to local antibiograms for resistance patterns, as well as individual patient risk factors for antibiotic-resistant pathogens (Table 1).[6,9,10]
Antibiotics
In general, previously healthy patients without serious comorbidities can be treated with a macrolide such as azithromycin or doxycycline. Patients who have recent use of antibiotics (typically within the last three months) or significant medical comorbidities such as chronic lung disease, end-stage renal disease, heart failure, or diabetes can be treated with a respiratory fluoroquinolone such as levofloxacin or a macrolide with a beta-lactam such as high-dose amoxicillin or amoxicillin-clavulanate.
In areas with high rates of macrolide resistant S. pneumoniae, the latter regimen is recommended for all patients regardless of comorbidities. This unfortunately encompasses many areas within the United States, so it is important for providers to be familiar with local resistance patterns. The guidelines also recommend prescribing oseltamivir/zanamivir for patients diagnosed with influenza A and whose symptoms have been present for less than 48 hours.[6]
For patients who are more ill and require admission to the hospital, guidelines recommend pursuing a similar antibiotic regimen as above with the addition of specifically-targeted antibiotics based on the patient's risk factors, including coverage for pseudomonas and MRSA as needed. This will often be based on the clinical gestalt of the treating physician.
Although the new IDSA-ATS recommendations for CAP have not yet been released, empiric antibiotic regimens will likely mirror the coverage for HAP and VAP as discussed below. Guidelines also recommend tailoring antibiotic therapy as soon as possible. This can be facilitated in the Emergency Department by sending blood cultures, rapid influenza tests, sputum cultures, tracheal aspirates and urinary antigens.
Unfortunately, the mortality of CAP has not significantly changed despite new antibiotics and guidelines since the advent of penicillin. Both the CDC and IDSA-ATS recommend influenza vaccine for all-comers and the pneumococcal vaccine for adults older than 65 years.[6,11] In the new era of growing pan-resistant organisms, it is likely that infection prevention will have greater effects on morbidity and mortality than infection treatment.
Hospital-Associated and ventilator-acquired pneumonia
As mentioned above, in late 2016, the IDSA released new guidelines for hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP), and did away with the concept of HCAP.[5] Hospital-acquired pneumonia is currently defined as a pneumonia not present at the time of admission that occurs 48 hours or more after entry to a hospital, while VAP is defined as pneumonia occurring 48 hours after endotracheal intubation.[5] Although more germane to inpatient management, these patients may be seen in the Emergency Department as individuals who present from long-term care facilities with ventilator dependence or who were recently discharged from a hospital.
The most recent IDSA-ATS guidelines stress choosing antibiotic coverage using the local antibiogram and blood and sputum cultures. However, if these are not readily known to the treating provider, empiric coverage should be initiated as discussed below and outlined in Figure 3. All patients who present to the Emergency Department with concern for HAP or VAP should be covered for common pathogens such as staph aureus, pseudomonas aeruginosa and gram-negative bacilli.[5]
Additional antibiotic choices are based on patient risk factors or clinical factors. In both HAP and VAP, the only data-proven risk factor for MDRO is the use of IV antibiotics in the past 90 days. However, other risk factors for MDRO include: septic shock upon presentation; acute respiratory distress syndrome (ARDS); the requirement for acute renal replacement therapy; 5 or more days of hospitalization prior to HAP/VAP development; and treatment in an ICU with known common MRDO isolates.[5]
Patients with these risk factors should be empirically covered for MRSA as well as pseudomonas. Even without these risk factors, patients should be empirically covered for MRSA in hospitals with 10-20% known rates of MRSA. Additionally, patients with significant underlying structural lung disease require additional pseudomonal coverage and MSSA coverage. The new guidelines support antibiotic treatment for at least seven days and to de-escalate as bacterial data becomes available.[5]
In sum, pneumonia is a challenging, multi-faceted disease process that requires early identification and appropriate antibiotic choice. Patients admitted to the hospital should be risk stratified based on individual risk factors and their presenting clinical symptoms. The emergency physician must be familiar with the local antibiogram to aid in choosing appropriate outpatient or inpatient medication regimens.
Authored by Susan Owens, MD Posted by Grace Lagasse, MD
References
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hosptial-acquired, ventilator-associated and healthcare-associated pneumonia. J Am Respir Crit Care Med. Feb 15 2005. 171 (4): 388-416.
- Kalil, AC et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Disease Society of America and the American Thoracic Society. Clinical Infectious Disease. 2016.
- Self, WH et al. Rates of emergency department visits due to pneumonia in the United States, July 2006-June 2009. Acad Emerg Med 2013 Sep: 20(9): 957-960 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3907184/
- Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014; 58:330.
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61. Available online at: http://www.idsociety.org/Guidelines/Patient_Care/IDSA_Practice_Guidelines/Infections_by_Organ_System/Lower/Upper_Respiratory/Hospital-Acquired_Pneumonia_(HAP)
- Mandell, LA et al. Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clinical infectious disease. 2007: 44. (Suppl 2).
- Calculator available online at: https://www.mdcalc.com/curb-65-score-pneumonia-severity
- “Outpatient vs. Inpatient Treatment of Community Acquired Pneumonia.” Ebell MH. Family Practice Management. April 2006:41-44; http://www.aafp.org/fpm/20060400/
- Wunderink RG, Waterer GW. Clinical practice. Community-acquired pneumonia. N Engl J Med 2014; 370:543.
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015; 386:1097.
- CDC summary of pneumonia vaccines avaialable online at: https://www.cdc.gov/vaccines/vpd/pneumo
- Available online at: http://pulmccm.org/main/2016/infectious-disease-sepsis-review/idsa-guidelines-2016-hap-vap-end-hcap-know-feel-fine/